Fortress Biotech Preferred $FBIOP
– Resumed dividends can create significant value for shareholders in the form of accumulated dividends and significantly increasing in share price
Disclaimer
This analysis represents only my personal opinions and is based on information that was current at the time of publication. The information is not intended as an investment recommendation or as advice from a certified financial advisor. All content in this analysis should only be used as a basis for further study and not as the sole basis for investment decisions. Investments in stocks and other securities always involve risk, including the possibility of losing some or all of the invested capital. Past performance does not guarantee future returns. The reader is encouraged to independently conduct a careful investigation and, if necessary, seek professional advice before making any investment decisions. I am not responsible for any consequences that may arise from using the information in this analysis, including inaccuracies or lack of updates.
The content herein is the exclusive content of Zellchair’s Substack, All Rights Reserved.
Quick Stats:
Name: Fortress Biotech Inc | 9.375% Series A Cumulative Redeemable Perpetual Preferred Stock (FBIOP)
Sector: Healthcare
Series: A
Redeemable: Yes
Perpetual: YeS
Cumulative: Yes
Pay Period: Monthly
Pay Dates: Last day of the month
Liquidation Preference: $25.00
Annualized Dividend: $2.343756
Original Coupon: 9.375%
Recent Market Price: $6.61 (2025-05-05 before market open)
Discount to Liquidation Preference: -$18.39 (-73.56%)
Recent Ex-Date: 6/14/2024
The background/description of the decision to pause the dividend:
“Across our portfolio, we could receive up to three regulatory approvals on NDAs and BLAs in the next 12 months for DFD-29, cosibelimab and CUTX-101, and potentially a fourth BLA filing as early as 2025 for CAEL-101. Based on its public statements, AstraZeneca has estimated that it expects the FDA to accept its BLA submission of CAEL-101 to treat AL amyloidosis for review as early as 2025, which could lead to approval and commercial milestone payments to Fortress. Additionally, Cyprium Therapeutics, our subsidiary company that developed CUTX-101, will retain 100% ownership over any priority review voucher that may be issued at NDA approval for CUTX-101. This is a pivotal time for the Company, and we are pausing the preferred dividend payments in order to maintain financial flexibility ahead of our multiple potential near-term milestones.” - Lindsay A. Rosenwald, M.D., Fortress’ Chairman, President and Chief Executive Officer (UNLOXCYT is today the brand name of Cosibelimab after FDA approval)
Important detail in the press release:
“In accordance with the terms of the Series A Preferred Stock, dividends on the Series A Preferred Stock will continue to accrue and cumulate until such dividends are authorized or declared.” - Source
Quick pitch:
In early July 2024, Fortress announced a pause in payment of dividends on 9.375% Series A Cumulative Redeemable Perpetual Preferred Stock. I believe that a couple of company-specific events will act as catalysts to reinstate the dividend to shareholders within 1-20 months. These triggers I believe will come from at least a couple of these:
I. Significantly reduced operating expenses
II. New restructured existing loans - Oaktree (Fortress Parent Company) & SWK (Journey Medical)
III. Subsidiary sold - Checkpoint Therapeutics (pending sale)
IV. Future CVR's - UNLOXCYT
V. FDA approval - CUTX-101 & CAEL-101
VI. Approvals outside the US - UNLOXCYT, EMROSI, CUTX-101 & CAEL-101
VII. Milestones from already completed deals - Cyprium Therapeutics (asset/drug CUTX-101 sold) & Caelum Biosciences (sold)
VIII. PRV Sale - CUTX-101
IX. New royalties from then approved drugs - UNLOXCYT (already FDA approved), CUTX-101 & CAEL-101
X. Significant sales growth in the already commercialized dermatology portfolio - Driven by the sales start (2025-03-24) of the Best-in-Class Oral drug for Rosacea in EMROSI
From today's 2025-05-05 share price of $6.61 until 2026-12-31, my estimates are for a share price of $22-$25 (+233-278%) and a total dividend of $5.8590 per share, which at today's share price corresponds to a dividend yield of 88.64%.
Note! I am a simple private investor who lacks both financial and medical education at university level. I have therefore not had the ambition to do a traditional financial analysis here, as I lack both the experience and the knowledge for that. I also want to mention that English is not my first language, so there is a risk of linguistic mistakes. This document/post I want only to be interpreted as a review of how I thought, reasoned and saw opportunities as risks with my personal investment in this specific case. Since my text is largely based on one company, I also advise all readers to read their own "Disclaimer" in their Investor Presentation material and read their "Risks" under “Part 1” in the latest Annual Report.
Table of Contents
1. Company
a. History & Structure
b. Business Model
c. Management
2. Ownership Structure
a. Common Stock
b. Preferred Stock
c. Public Subsidiaries
d. Private Subsidiaries
3. Overview of Portfolio/Pipeline
a. Preclinical
b. Phase 1
c. Phase 2
d. Phase 3
e. Commercial Portfolio
4. Public Subsidiaries
a. Checkpoint Therapeutics
b. Journey Medical
c. Mustang Bio
d. Avenue Therapeutics
5. Private Subsidiaries
a. Cyprium Therapeutics
b. Urica Therapeutics
c. Helocyte
d. Oncogenuity
e. Cellvation
6. Subsidiaries Deals
a. Checkpoint Therapeutics
b. Urica Therapeutics
c. Cyprium Therapeutics
d. Caelum Biosciences
e. AEVITAS
f. Avenue Therapeutics
7. Financial
a. Revenue
b. Operating Expenses
c. Debt
d. Dividend
e. Future Expected Revenues (2025-2026)
f. Future Expected Savings (2025-2026)
g. Financial Summary
8. Closest comparable peers/stocks
a. Milestones & Royalty-based Companies
b. Investment Companies in part-ownership
9. Risks
10. Why (I believe) Fortress Biotech Preferred Stock will resume the dividend within 1-20 months
11. Valuation, Conclusion & Position
1. Company
a. History & Structure
Founded 2006 under the name Coronado Biosciences, initially as an oncology research company. In 2011 they changed their business model to acquire medical assets to further take the development forward under its own direction when they raised $47.4M in funding. Shortly thereafter it became a public company by registering all its private shares as common stock. Later in 2015, when the most assets for the start-up of their subsidiaries were acquired and the change in the business model was completed, they changed their name to Fortress Biotech.
Fortress Biotech is a biopharmaceutical company engaged in acquiring, developing and commercializing new pharmaceutical and biotechnology products. Fortress provide financing, board and management services to each of the subsidiaries and seek licensing, partnerships, joint ventures and/or public and private funding for research and development. Fortress subsidiaries have a broad portfolio in several different therapeutic areas including dermatology, oncology/haematology, pain management, rare diseases and others.
Current Partner Company/Subsidiary – Founding (effective year for inclusion in Fortress Biotech’s portfolio)
Journey Medical – 2014
Checkpoint Therapeutics – 2015
Mustang Bio – 2015
Avenue Therapeutics – 2015
Helocyte – 2015
Cellvation – 2016
Cyprium Therapeutics - 2017
Urica Therapeutics - 2017
Oncogenuity – 2017
Class of Stock - Outstanding Shares (2025-03-27)
Fortress Biotech Common Stock $FBIO ($0.001 par value) - 29,533,840 shares
9.375% Series A Cumulative Redeemable Perpetual Preferred Stock $FBIOP ($0.001 par value) - 3,427,138 shares
b. Business Model
Fortress has a unique model from its founding that differs from its closest peers. Namely, that it has founded all its subsidiaries entirely by itself, with the exception of Journey Medical, where there is also a co-founder. Fortress management has historically acquired medical assets with the aim of creating niche subsidiaries around these assets. This is with the aim of creating internal diversification, clarification, being able to increase/decrease focus and financing depending on the development of the subsidiaries and being able to create the best suited teams for knowledge against specific diseases and drugs in these subsidiaries. With this background Fortress also had the opportunity to become the majority owner in its subsidiaries, as it was the fact they who founded them, with the aim of controlling future decision-making by controlling all outstanding Common A-shares or Common Preferred A-shares in the subsidiaries. Fortress was also smart to create more incentives for future development with future royalties from sales (usually 4.5%) and annual equity dividend (usually 2.5%) from the subsidiaries.
According to Rosenwald, “the model would take the best attributes of a royalty business, private equity, and traditional biopharma to focus primarily on clinical development and commercialization, without the risk of extensive research and preclinical development”. (Source)
Fortress Biotech believes this model has the potential for snowballing growth as they acquire new assets and as their subsidiary/partner companies grow in value.
Fortress Biotech Company Webcast @ Lytham Partners Fall 2024 Investor Conference (Q3/Q4 2024) -
c. Management
Management in Fortress has founded, built, managed and sold pharma/biotech companies for several billions before starting Fortress Biotech. I want to highlight three people from management and what they have historically accomplished in the industry.
Lindsay A. Rosenwald, M.D. (Founder, Chairman, President and Chief Executive Officer)
From 1991 to 2008, Dr. Rosenwald served as the Chairman of Paramount BioCapital. Over the last 26 years, Dr. Rosenwald has acted as a biotechnology entrepreneur and has been involved in the founding and recapitalization of numerous public and private biotechnology and life sciences companies. To cite some examples:
Companies
Indevus Pharma
Co-Founded Indevus Pharma in 1988 which was bought out by Endo Pharmaceuticals in 2019 for $370M
Chelsea Therapeutics
Co-Founded Chelsea Therapeutics in 2002 which was then sold in 2014 to Lundbeck for $658M
Cougar Biotechnology
Co-founded Cougar Biotechnology in 2003, which just a few years later was bought out by J&J itself for $1B
Drugs
ZYTIGA
“… Zytiga at Johnson & Johnson, which is the biggest selling prostate cancer drug in the world. When I identified it for Cougar Biotechnology, it was being developed by a small British company. There were no patents, but we knew that it had dramatic effects on prostate-specific antigen levels at the end-stage. Before market exclusivity ended, it was generating annual revenue of about $4 billion.”
NORTHERA
“… Northera for dizziness and lightheadedness in patients who have Parkinson’s. We found out that it was being successfully used in Japan to treat highly untreatable neurogenic orthostatic hypotension patients, and there was no patent. We bought the data from the company developing it, set up Chelsea Therapeutics, got it approved, and sold it to Lundbeck for about $600 million.”
TRISENOX
“… Trisenox for acute promyelocytic leukemia, a form of leukemia that was incurable before the drug came on the market. It was being used in China and was highly effective. Then we found a researcher at Memorial Sloan Kettering Cancer Center who was already exploring it. We ultimately bought worldwide rights to the drug from the institution and completed the FDA approval process in record time, about two-and-a-half years.”
(Source for the three quotes above)
Here are some interviews/presentations from last year with Lindsay Rosenwald that I can recommend:
Linsday Rosenwald from Fortress Biotech - StockOcean Interview -
Is This "Biotech Winter" Worse Than Others? Stocks To Watch –
Biotalk Episode 18: A Conversation with Lindsay Rosenwald of Fortress Biotech – Source
Michael S. Weiss (Executive Vice Chairman, Strategic Development)
Weiss managed Paramount Capital/Aries Funds alongside Rosenwald and later went on to form Access Oncology which was later acquired/merged by Keryx Biopharmaceuticals in 2004. Following the merger Weiss remained as CEO of Keryx for a couple of years.
Founded TG Therapeutics (TGTX) in 2011 and joined Fortress Biotech 2014 in preparation for the acquisition of several medical assets and the formation of subsidiaries.
Weiss TG turned $31M into multi-billion valuation:
Manhattan Pharmaceuticals and their majority-owned subsidiary TG Therapeutics, with early expertise in antibody-dependent cell cytotoxicity (ADCC), receive a call in 2011 from LFB Group in France that their LFB wholly-owned subsidiaries LFB Biotechnologies and GTC Biotherapeutics need help presenting a poster on 53rd ASH Annual Meeting, San Diego USA, in December 2011 (Source).
Cooperation leads to, months later:
"TG Therapeutics Completes Licensing Agreement with LFB Biotechnologies for the Development of Ublituximab" (Source)
The deal leads to a name change and preparation for the IPO:
"Manhattan Pharmaceuticals, Inc. Announces Name Change to TG Therapeutics, Inc. and Reverse Stock Split" (Source)
In TG 2013 annual report, the financial details of the Ublituximab deal are revealed for the first time:
"In January 2012, we entered into an exclusive license agreement with LFB Biotechnologies, GTC Biotherapeutics, and LFB/GTC LLC, all wholly owned subsidiaries of LFB Group, relating to the development of TG-1101. Under the license agreement, we have acquired the exclusive worldwide rights (exclusive of France/Belgium) for the development and commercialization of TG-1101 (ublituximab). To date, we have made no payments to LFB Group and LFB Group is eligible to receive payments of up to an aggregate of approximately $31.0 million upon our successful achievement of certain clinical development, regulatory and sales milestones, in addition to royalty payments on net sales of TG-1101 at a royalty rate that escalates from mid-single digits to high-single digits.” (P. 12, Source)
Today years later, TG-1101 (ublituximab) is TG first and to date only approved product with the brand name BRIUMVI, and at the time of writing the stock is valued at $6.86B. Weiss owns approx. 9% TGTX today, and to my knowledge he has never sold a single share (other than a few due to tax reasons). So far, the return on that $31M described above has generated a 22061% return on the share price and a CAGR of approximately 57% per year from 2012 (when the acquisition was completed) through 2024.
Another one who was around throughout whole this era of TG Therapeutics was Director Neil Herskowitz, and to make the Fortress Biotech connection again, today he sits as a Director of all FBIO's publicly traded subsidiaries)
Rosenwald and Weiss also started Opus Point Partners a Hedge Fund managing a long/short equity that focused on the healthcare and biotechnology sectors in 2008. The fund closed down around 2019, most likely to focus only on Fortress and TG Therapeutics and the moral dilemma of standing on the buy/sell side of now direct colleagues in the industry or competitors/hedges to their respective companies and pipelines.
George Avgerinos, Ph.D (Senior Vice President, Biologics Operations)
First BASF Bioresearch, Abbott Laboratories and last AbbVie, where he was Vice President, HUMIRA Manufacturing Sciences and External Partnerships. In his 22-year career at AbbVie he supported expansion of the supply chain to over $9.0 billion in annual global sales. Humira then became the world's top-selling pharmaceutical in the world. For this, Dr. Avgerinos received several awards.
2. Ownership Structure
Here is the latest update of Fortress Biotech's ownership in percentage:
(Note that this is from 2024-12-31, and since Fortress is awarded its regular 2.5% Annual Equity Dividend starting from January 1st of each year, the ownership may be even higher today unless dilution with new shares has occurred)
a. Common Stock
Insider Ownership
Insider buys in Common Stock (FBIO) have been massive in relation to the company's market value and despite their high initial ownership:
Some buys that stand out:
Who – When – Shares Purchased – Stock Price - Total Cost
Rosenwald – 2024-09-23 - 763,359 - $1.84 - USD 1,404,58
Rosenwald – 2023-11-14 - 1,567,515 - $1.70 - USD 2,664,776
Weiss – 2023-11-14 - 147,058 - $1.70 - USD 249,999
Rosenwald – 2023-02-10 - 2,395,209 - $0.84 - USD 2,000,000
Weiss – 2023-02-10 - 1,197,604 - $0.84 - USD 999,999
In Q3 2024, Highbridge Capital Management purchased 2,734,854 shares of Common Stock issuable upon the exercise of warrants, which then increased their ownership in Fortress Common Stock to 9.0% (Source).
b. Preferred Stock
I have not seen any figures on whether and if so, how many shares, any insiders owned at the introduction in 2017. Despite the Founder/President/CEO's Lindsay Rosenwald large holdings of Common Shares (FBIO), he has purchased 139,167 shares of the Preferred Stock (FBIOP) worth $2,530,295M in total with an average acquisition value of $18.18 per share. Note that he bought for $1M on the Liquidation Preference value of $25 per share, and all well above today's share price of $6.61.
Buy in order of size (shares – share price – price paid)
40,000 - $25.00 - USD 1,000,000
52,500 - $18.00 - USD 945,000
16,667 - $18.00 USD 300,006
10,000 - $11.75 - USD 117,500
10,000 - $9.34 - USD 93,400
5,000 - $7.39 -USD 36,971
5,000 - $7.35 - USD 37,418
c. Public Subsidiaries
Here you can of course review the ownership pictures yourself based on your most suitable available tools. I just wanted to point out that in addition to Fortress management's high insider ownership in FBIO/FBIOP, they also own some shares privately in these subsidiaries, partly through options but also purchases over the market. This applies primarily to Rosenwald and Weiss.
In Journey Medical (DERM) I also want to draw your attention to the fact that the notorious (in small cap biotech) Kevin Tang and his Tang Capital Management have recently taken a larger position, for better or worse. Better because he is recognized as good at bottom fishing for cheap stocks, like Journey. Worse or better (from previous experience) that he has acted as an "activist" investor in a couple of companies and can create (in my opinion) concerns among a board of directors and the stock's private investors. However, it should be said that in most cases he is more of a useful demander who is also not afraid to make buyout offers for small and undervalued companies. (Source)
d. Private Subsidiaries
It is more difficult to get an overview here of course with considerably less public information. I think the same applies here, however, that at least Rosenwald and Weiss may own some privately or via own companies. According to an old list of owners in Cyprium PPS (more on that further down) judging by what I came across earlier, it looked like there were some private individuals and smaller Family Offices. I don't think it is a wild guess that these have good contact with Rosenwald privately or professionally and have been involved in one or more of his previous successful deals. But I can neither confirm nor vouch for this.
3. Overview of Portfolio/Pipeline
This is the idea to show what level a drug is/was at at the last update.
a. Preclinical (7/39)
AAV-ATP7A (AAV Gene Therapy) - Menkes Disease (CUTX-101 2.0)
MB-109 - Recurrent GBM and anaplastic astrocytoma (MB-101+MB-108)
CK-103 – BET Inhibitor for Myelofibrosis and Solid Tumors (Single Agent)
CK-302 - Anti-GITR for Solid Tumors (Single Agent/Combination)
CK-303 - Anti-CAIX Renal Cell Carcinoma/Solid Tumors (Single Agent/Combination)
AVTS-001 (AAV.SFH Gene Therapy) - Complement-mediated diseases (Asset sold to 4DMT in April 2023)
CEVA-102 (Cell Therapy) - TBI, GvHD, ARDS, CHF, Crohn’s (Off-the-Shelf)
b. Phase 1 (11/39)
Dotinurad* - URAT1 Inhibitor for Gout
MB-106 - CD20 CAR-T for autoimmune
Olafertinib - Frontline NSCLC with EGFR Mutations
Olafertinib + Cosibelimab (UNLOXCYT) – Second line NSCLC EGFR Mutations
AJ201 - Spinal and bulbar muscular atrophy (Kennedy’s Disease) P1b
MB-101 - IL13Rα2 CAR-T for recurrent glioblastoma (GBM)
MB-108 - HSV-1 oncolytic virus (OV) for recurrent GBM
Triplex- Allogeneic (Haplo, Mismatch) Stem Cell Transplant in Peds
Triplex - Combo with CD19 CAR for NHL
Triplex - CMV-HIV CAR (+/- Triplex)
BAER-101 - Rare epilepsies
c. Phase 2 (6/39)
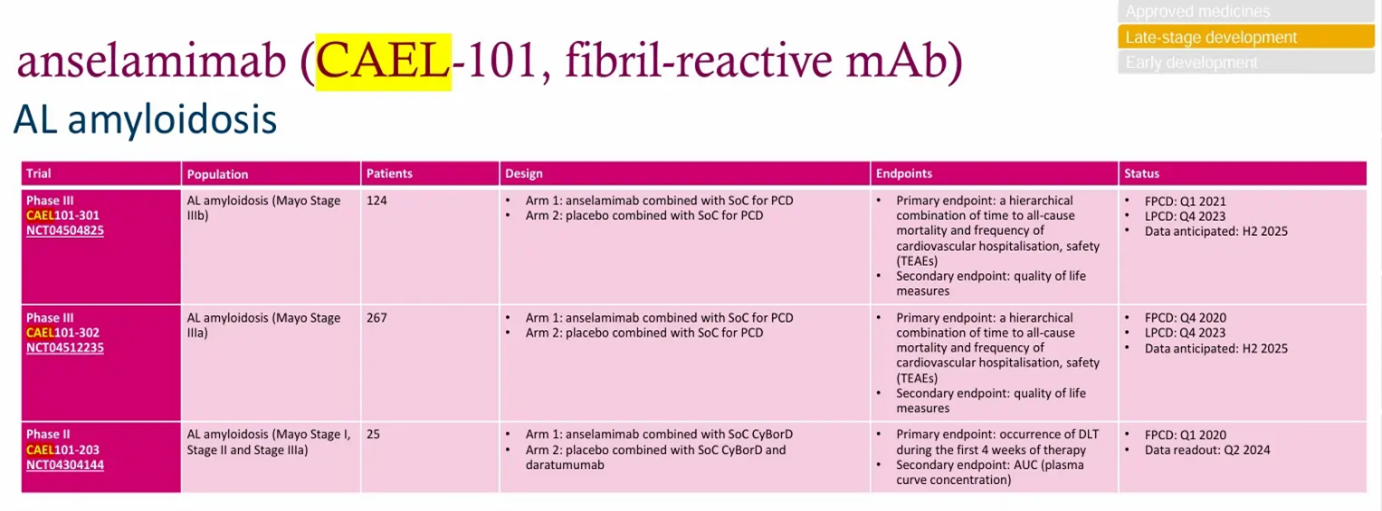
AJ201 - Spinal and bulbar muscular atrophy (Kennedy’s Disease) P2a
CAEL-101 - Light chain (AL) amyloidosis (Stage I, II & IIIa) + cyclophosphamide-bortezomib-dexamethasone (CyBorD) chemotherapy & daratumumab
Triplex - Liver Transplant (Recipient Vaccination)
Triplex - Kidney Transplant (Recipient Vaccination)
Triplex - CMV-HIV Co-Infection on ART
Triplex - Allogeneic (MRD) Stem Cell Transplant (Donor Vaccination)
d. Phase 3 (4/39)
CUTX-101 - Copper histidinate for Menkes
CAEL-101 - Light chain (AL) amyloidosis (Stage IIIa) + SoC
CAEL-101 - Light chain (AL) amyloidosis (Stage IIIb) + SoC
IV Tramadol - Post-operative acute pain management
e. Commercial Portfolio (10/39)
QBREXZA - Primary axillary hyperhidrosis (excessive sweating)
ACCUTANE - Severe recalcitrant nodular acne
EMROSI - Inflammatory lesions of rosacea
UNLOXCYT - Metastatic Cutaneous Squamous Cell Carcinoma (cSCC)
UNLOXCYT - Locally Advanced Cutaneous Squamous Cell Carcinoma (cSCC)
AMZEEQ- Inflammatory lesions of non-nodular acne
ZILXI- Inflammatory lesions of rosacea
EXELDERM - Antifungal cream for common skin infections
TARGADOX - Adjunctive therapy for severe acne
LUXAMED - Water-based emulsion for wounds
Unknown Stage – (1/39)
CEVA-D - Mechano-transduction Device for Cell Therapies
*Dotinurad - Dotinurad has been approved in several Asian countries since 2020, where two pivotal P3s are now planned in the US, so "judging" it as a Phase 1 drug is probably misleading (but more on that below).
This review will directly or indirectly focus on the marked assets due to triggers in the form of financial savings, sales or other growth opportunities for the time span May 2025-FY2026. Of course, this does not necessarily mean that more assets may have an impact during this time span (positively or negatively).
4. Public Subsidiaries
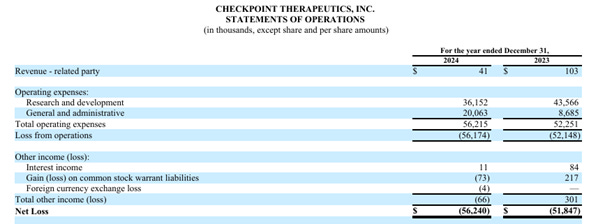
a. Checkpoint Therapeutics
Brief description: Checkpoint Therapeutics is a biopharmaceutical company focused on immunotherapy in oncology, and on acquisition, development and commercialization of new treatments for patients in a more effective and potentially more affordable way.
Ownership: 8.3%
Annual Equity Dividend: 2.5%
Royalty: 4.5%
FDA Approved asset: UNLOXCYT (Q4 2024)
Pipeline: Olafertinib, CK-103, CK-302, CK-303
Comments: Read more under "Subsidiaries Deals" below.
b. Journey Medical
Brief description: Journey Medical is focused on identifying, acquiring and strategically commercializing innovative, differentiated dermatology products through its efficient sales and marketing model.
Ownership: 44.5% (Co-Founder & CEO Claude Maraoui 13.5%, COO Ramsey Alloush 3.5%, Lindsay Rosenwald 2.2%)
Approved assets: QBREXZA, ACCUTANE, EMROSI, TARGADOX, AMZEEQ, ZILXI, EXELDERM, LUXAMEND
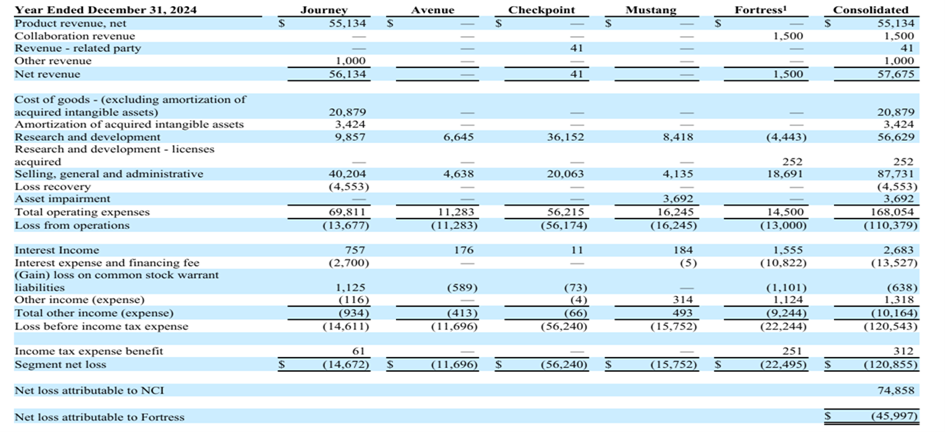
Total Sales 2024: $56M
Comments: Claude Maraoui has more than 30 years of experience in commercializing some of the most successful dermatology products in the world. He served as Vice President of Dermatology Sales at Medicis Pharmaceutical Corporation and has held various leadership positions in marketing and sales for therapeutic and aesthetic dermatology. He has executed more than 50 product launches during his career. Almost the entire Management (6/8) consists of former high-ranking executives from Maraouis former Medicis Pharmaceutical. Medicis were known for products such as SOLODYN and ZIANA for treating Acne, and for RESTYLANE and DYSPORT for treating facial wrinkles. In 2012 Medicis was acquired by Valeant Pharmaceuticals International for $2.6B.
8 marketed commercial products in Dermatology Therapeutics from Journey Medical is available today
QBREXZA - First and only prescription cloth towelette approved to treat excessive underarm sweating (primary axillary hyperhidrosis
TARGADOX - Indicated as adjunctive therapy for severe acne. It is the smallest branded doxycycline tablet available in the United States.
EXELDERM - Available as an antifungal cream or solution that helps relieve symptoms of common skin infections: ring worm, jock itch, and tinea versicolor. EXELDERM Cream can also help relieve the symptoms of tinea pedis.
LUXAMED- Water-based emulsion formulated to provide an optimally moist healing environment for superficial wounds; minor cuts or scrapes; dermal ulcers; donor sites; first- and second-degree burns, including sunburns; and radiation dermatitis.
ACCUTANE - Used to treat a type of severe acne (nodular acne) that has not been helped by other treatments, including antibiotics.
AMZEEQ - First and only FDA-approved topical formulation of minocycline for the treatment of inflammatory lesions of non-nodular moderate to severe acne vulgaris in patients 9 years of age and older.
ZILXI - First and only FDA-approved topical minocycline for the treatment of inflammatory lesions of rosacea in adults.
EMROSI – Indicated to treat inflammatory lesions (papules and pustules) of rosacea in adults.
Journey's historical plan in a growth model with increased risk
1. Acquire cheap dermatology products where patent protection was about to expire to squeeze out the last sales of these products. This is due to the low upfront and royalty costs where it was calculated that the products would be outcompeted by generics in almost all cases.
2. With point 1, they could slowly but surely build a sales team in addition to those who came with them from previous Medicis.
3. Acquire more expensive and more established products.
4. Acquire clinical products (DFD-29/EMROSI) that have proven themselves in humans (i.e. after P1 at the earliest).
5. Start selling outside North America via an out-licensing model (QBREXZA, AMZEEQ)
Journey Medical - Total Sales
2016: $3M
2017: $15M
2018: $23M
2019: $34M
2020: $44M (start of the generic infringements/competition within “others”)
2021: $63M
2022: $73M
2023: $79M (QBREXZA $19M upfront sales deal included)
2024: $56M
With this strategy, despite almost maximum generic counter-effect, Journey has not only managed to maintain sales but has also increased them. I know those who, in writing or speaking, both from investors and analysts, have shown certain question marks and concerns about declining or stagnant sales in certain products. Here I think Journey has been clear during presentations, quarterly reports and conference calls. In line with the ongoing success they could see from EMROSI's study results, they have continued to drastically cut back on the sales team in recent years despite the major downsizing in the team in 2020–2021, when they expected the first generic competitors to reach the market. They have also stopped marketing a couple of products to save money and in doing so have let certain products "die on their own" despite probably having a few million left in sales despite generic competition.
The goal has been simple on paper, to have as good a financial balance and as few diluted shares as possible on the day EMROSI reaches the market. As described above, the effort to get there has been difficult at times in reporting declining or stagnant sales figures for several products. In the long term I believe it has been the right strategy and something that the shareholders, regardless of whether they are owners of Fortress and/or Journey, will be rewarded for in the long run. Journey has so far had the “snowball-effect”, and with EMROSI now as a commercial drug, I believe the snowball has every opportunity to expand in both size and speed.
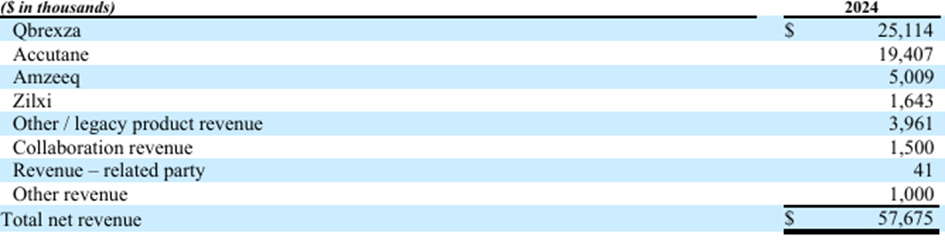
ACCUTANE & QBREXZA
Some numbers that describe the success of ACCUTANE and QBREXZA:
Journey's upfront payment for ACCUTANE was $1M. In less than 4 years, it has grown to have a 16% market share in the Isotretinoin market in the US.
Before Journey acquired QBREXZA, Dermira spent $82.8M in patient advertising during the 3-year period they promoted QBREXZA. Under Journey’s ownership, QBREXZA has grown TRx by over 58% in 3 years, spending less than 3% of Dermira’s commercial budget.
ACCUTANE
2021: $10.053M
2022: $18.373M
2023: $20.168M
2024: $19.407M
QBREXZA
2021: $17.056M
2022: $26.715M
2023: $25.410M
2024: $25.114M
(QBREXZAS $19M upfront sales deal 2023 not included)
Since 2024, ACCUTANE (from Q2-Q3) and QBREXZA (Q4) have also faced increased competition in the domestic US market. This was addressed in the Q&A during the conference call last quarter (Q4 2024):
“Scott Henry, Analyst, AGP: Okay. So none of the products you would say have kind of reset at lower levels. I mean, mostly I’m thinking QBREXZA and ACCUTANE are the main drivers there.
Claude Maraoi, Co-Founder, President and CEO, Journey Medical: Yes. QBREXZA continues to perform well for us. We continue to see script growth year over year. And it’s holding very solid. It obviously has some new competition out there. But again, the prescriptions continue to show very good positive signs. Again, when you look year over year, we’re growing that product. With ACCUTANE, as we’ve stated in the past, there were a couple new entries towards the second and third quarter of twenty twenty four. We did see some slowdown with ACCUTANE. We’ve stabilized that business relatively well and we believe we’re going to have good performance with ACCUTANE”
Other commercial assets
Sales – “Others”
2017: $15.5M
2018: $23.4M
2019: $34.9M
2020: $44.679 M (Start of the generic infringements/competition)
2021: $36.358M
2022: $25.907M
2023: $14.082M
2024: $10.613M
As you could see everything hasn’t been a straight line and a success story, especially not in cases where the generic risk was included but where new and better dermatological products also reached the market faster than expected. However, the choice of model and that it was delivered well is today reassuring but historically expected as the management has done this before, and also to a large extent even then under the same common roof for Medicis. Although not all of them sat in the highest-ranking roles in the management/board of Medicis, 6 current people in the management were responsible for almost the entire commercial team. In other words, they are experts in sales with experience of over 50 commercialized products in the US. With the addition of Joseph Benesch in finance & M&A and Srinivas Sidgiddi (EMROSIS's father who joined in connection with the acquisition from Dr. Reddy) in research, product development and clinical studies. Journey is now a complete team both on paper and based on the words of co-founder CEO Claude Maraoui. This is of course timely now when the company is facing a possible paradigm shift. Let me introduce EMROSI.
EMROSI
2021 Journey acquired global rights to EMROSI for Rosacea, including in the U.S. and Europe, except that Dr. Reddy’s Laboratories has retained certain rights to the program in select markets including Brazil, India, China and the Commonwealth of Independent States (“CIS”) countries Russia, Armenia, Azerbaijan, Belarus, Kazakhstan, Kyrgyzstan, Moldova, Tajikistan and Uzbekistan.
Under the agreement Journey and DRL would collaborate to monitor two P3s in the US and Germany, as well as a P1 aimed at generating more discoveries. In the previous P2 in Germany EMROSI had already demonstrated statistical significance over both placebo and active control, ORACEA (doxycycline, Galderma), on both co-primary endpoints. In fact, EMROSI had about double the efficacy when compared against ORACEA previous study results for both endpoints. And here it should be remembered that ORACEA had been unchallenged as an oral alternative for Rosacea since their FDA approval in 2006, i.e. for two decades. But how would EMROSI manage to follow this up in two extended studies that would also be head-to-head against the Standard of Care ORACEA? The study results in P3 did not come in line with P2, they were even better than that. I am not going to require you to review every word and number in the study results, but, if you have made it this far and want to continue reading, you almost have to go through Journey Medical's Corporate Overview to get a thorough overview of the results. (Source)
I hope you also want to hear how exciting these study results were for Journey themselves. Then listen to this Investor Presentation during the Planet MicroCap Showcase Conference in Las Vegas last year (recommend the whole thing, but DFD-29/EMROSI specifically between 12:50–20:30):
In November 2024, EMROSI was granted FDA Approval
Claude Maraoui, Co-Founder, President, and Chief Executive Officer of Journey Medical
“With approval from the FDA, Journey Medical is proud to deliver Emrosi, a unique treatment option for the millions of patients in the U.S. suffering from rosacea. Rosacea is a difficult to treat skin condition and based on the favourable results from our Phase 3 clinical trials, Emrosi has potential to become the best-in-class oral medication to treat the condition. Our seasoned dermatology-focused sales force is now preparing for a successful launch and to establish Emrosi as a new standard of care in the treatment of rosacea. Journey Medical is committed to bringing cutting-edge innovation to patients with dermatological conditions and the healthcare professionals who treat them.” (Source)
And of course, companies (perhaps especially in healthcare) are/can be biased, so you should always listen to the leading expertise in the field. The problem here? When the "Rosacea elite" in the US, who are heard at the major conferences and get to write/publish in the Medical Journals, saw the P2 result almost everyone seemed to want to be part of the P3 study when EMROSI entered the US via Journey (honour and glory for Linda Stein Gold, Joshua Zeichner, Zoe Diana Draelos, James Q Del Rosso and others).
"Emrosi offers a new treatment option, mainly for the pimple-like bumps, by reducing swelling and inflammation. Each capsule contains 40 milligrams of minocycline, including 10 milligrams that work immediately and 30 milligrams that are released slowly over time (called extended release). This helps maintain a steady level of the drug in the body over a longer duration and provides better results. It also means fewer daily doses and a lower risk for side effects." (Source)
"Minocycline from DFD-29 with its modified-release formulation provides higher dermal concentration than doxycycline from Day 1 onwards at a similar dose. The higher concentration from Day 1 at the site of action is expected to translate into a clinically meaningful impact in the treatment of patients with rosacea." (Source)
“The approval of Emrosi adds another arrow to the quiver for physicians targeting their patients’ papulopustular rosacea and may be of special interest to patients concerned about antibiotic resistance because of its narrow spectrum of antibiotic activity.” (Source)
“A Breakthrough Rosacea Treatment with Long-Lasting Effects for Adults” (Source)
"The approval of DFD-29 signals a shift in the treatment paradigm for rosacea, with Emrosi anticipated to become the best-in-class oral therapy for the condition." (Source)
“Not only does it prove the efficacy of minocycline, it is safe for the long-term per the study data. Patients were really happy. Nobody wanted to give it up" (Source)
"If Journey Medical effectively communicates Emrosi’s clinical advantages and secures favourable market access, the drug has the potential to become a leading treatment option for rosacea, improving outcomes and quality of life for patients.” (Source)
"The drug demonstrated statistically significant superiority over both Galderma’s standard-of-care Oracea” (Source)
“The launch will mark a change in the treatment landscape. GlobalData’s 2022 ‘Rosacea Marketed and Pipeline Drugs Assessment, Clinical Trials and Competitive Landscape‘ report stated that Galderma was ‘the most prominent player in the rosacea space, offering a comprehensive portfolio of solutions’. However, it also warned that with “no presence in the late-stage pipeline and with its existing marketed products widely genericised, the company may lose its position in the rosacea market” (Source).
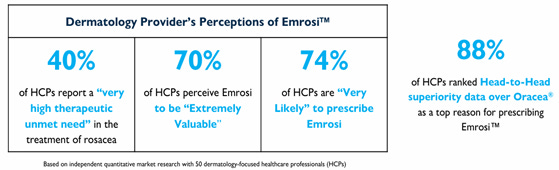
Even before EMROSI received its FDA approval, Journey was eager and smart enough to try, if possible, to get a first glimpse of the market picture of what the future might look like with EMROSI approved in the US. They enlisted help of an external survey company which collected survey results from HCPs in the country to compile into a report. The results were unheard of for the drug with several months to go before PDUFA (from Q1 2024 conference call transcript):
“Healthcare prescribers overwhelmingly confirmed their willingness to adopt and prescribe DFD-29 for the Rosacea patients, and a adoption rate of 79%. In our industry, this is an astoundingly high rate and translates into an approximate 8 out of 10 Rosacea prescriptions going to DFD-29. This rate exceeded even our own internal expectations and gives us the confidence in a successful DFD-29 launch.”
“Given the 16.5 million Rosacea sufferers in the United States and over four million prescriptions written annually, the prescriber adoption rate captured in our market research makes us even more excited about DFD's market potential.”
“With respect to the payer market research results, the data shows most, if not almost all PBMs, GPOs, and other managed care organizations are likely to contract with us to provide coverage for DFD-29 for over 200 million lives.”
“As a result of this market research, we are even more confident that DFD-29 will have high acceptance among prescribers and that negotiations with payers for formulary inclusion and reimbursement will be favorable after DFD-29 is approved. We will provide additional details on pre-launch activities later in the year as we finalize the launch plans, pricing, and product positioning for DFD-29.”
Robert Nevin (Chief Commercial Officer) topped this on the “EMROSI – U.S. Commercial Conference Call” in early February this year when he described what commercial opportunities he had to work with, now with EMROSI, which had never been better than this in his entire career. And remember that he was responsible for the commercial strategy for what was then some of the world's best-selling products in therapeutic dermatology history with brand names like SOLODYN, ZIANA, RESTYLANE and DYSPORT. This largely due to EMROSI’s superiority of SoC ORACEA and an award-winning sales team behind him.
Last quarter, Journey's sales team consisted of only around 35 people, but, given the market landscape, they will initially be able to cover a large part of the need. They have expressed during previous conference calls that over time they expect to gradually increase the ceiling somewhere around 40-45 people. To put a figure on this, management believes that they can quickly reach the top 80% of TRx prescriptions with the current sales team. And of these top 80% of TRx, 92% already prescribe at least one of Journey's previous products.
CEO Maraoui’s two specific sentences from the Las Vegas presentation still echo in my head:
“I will be able to look patients in the eye and say we have placebo-like side effects”
“We will be the new Standard of Care”
If you have superior results, you should also be able to sell at a premium to existing significantly inferior alternatives. During the commercial launch call, these figures were reported:
ORACEA Brand - $914
ORACEA AG -$783
EMROSI - $1298
In response to a direct question from me at an Investor Conference last year, CEO Maraouis answered “Yes” to the question of whether EMROSI will do $50M in sales in the first year (trailing twelve months). This should be compared to the $56M in sales that the remaining product portfolio in Journey did in total in 2024. It should also be noted that Journey themselves have stated on more than one occasion that they see the major increase in sales starting at 9-12 months in when sales subscription and coverage of EMROSI start to gain momentum.
When ROTH MKM analysed Journey in August 2024, they assumed that the price for EMROSI would be in line with ORACEA's price, and progressed the following sales development for EMROSI in the coming years:
2025
Q1: $0M
Q2: $5M
Q3: $14M
Q4: $21M
Total: $40M
2026
Total: $80M
2027
Total: ?
2028
Total: $200M
If they received $19M cash upfront in a sales deal (up to $45M in total) in parts of Asia for QBREXZA, what can EMROSI bring in (excluding BRIC and CIS)? A deal that includes, for example, the entire EU and UK? When it comes to sales deals for both EMROSI and other assets in the portfolio, Journey has been willing to point out in every conference call or investor presentation that they are in active discussions with several partners for various possible deals.
ROTH MKH latest Estimated EPS for Journey Medical (April 3, 2025), where it lowered its forecasts for ACCUTANE and QBREXZA given the increased competition mentioned above:
2026: $2.18 EPS
2027: $3.32 EPS
2028: $5.18 EPS
P/E based on todays $7.03 in DERM (2025-05-05) share price:
2026: P/E 3.22
2027: P/E 2.11
2028: P/E 1.35
Final Comments: The CEO explained at the 37th Annual ROTH Conference in Q1 2025 that EMROSI will become the new Standard of Care (over 50% prescription within its niche), given its superiority in head-to-head P3 clinical trials against current SoC ORACEA, with good potential of +$200M in US and +$100M outside the US (with partners) per year sales around peak sales. Add this, and +70% margins, to the bottom line to today's flat results and it should spike the market value from here significantly in the future. If Fortress, CEO, COO, Tang other insiders intend to keep all their shares, the free float is already low with potentially narrower openings for larger investors than alleys in an old Italian town.
If you want more thoughts and analysis on Journey Medical, I can recommend these:
Journey Medical FDA Approval For Emrosi Makes This An Exciting Stock (Source)
Journey Medical Coorporation Emrosis Launch Could Be A Turning Point (Source)
Journey Medical Corporation: Why It Might Be Worth Having A Skin In This Game (Source)
Journey Medical: A Small-Cap Multibagger Hiding in Plain Sight (Source)
c. Mustang Bio
Brief description: Mustang Bio is Pioneering Innovative CAR-T Therapies for Cancer and Gene Therapies for Primary Immunodeficiencies.
Ownership: 6.3%
Annual Equity Dividend: 2.5%
Royalty: 4.5%
Pipeline: MB-101, MB-106, MB-108 & MB-109 (MB-101+MB-108=MB-109)
Comments: First, read about Mustang Bio and uBriGene Biosciences' initial deal and then the collapse of that same deal to get an eye-opener on the big reason Mustang ended up where it is today, despite compelling clinical study results. Now the wait-and-see mode looks set to continue until Q4 unless the company or any of its assets are sold before then:
MB-106
“… further clinical development of MB-106 is currently focused solely on autoimmune diseases unless funding and resources become available to restart the program for hematologic malignancies. Planning for the aforementioned Phase 1 investigator-sponsored clinical trial in autoimmune diseases is in progress, with initiation anticipated in the fourth quarter of 2025.” – P.12, Annual Report 2024
MB-109
“In October 2023, we announced that the FDA accepted our IND application for the combination of MB-101 and MB-108 – which is referred to as MB-109 – for the treatment of patients with IL13Rα2+ relapsed or refractory glioblastoma (“GBM”) and high-grade astrocytoma. Pursuant to termination of the lease of our cell processing facility in Worcester, MA, we are exploring with COH and Nationwide the possibility of initiating this clinical trial as an investigator sponsored single-institution study at COH in the fourth quarter of 2025.” – P.6, Annual Report 2024
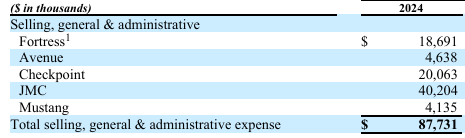
Due to the company's current financial and therefore medically uncertain status and declining clinical trial activity, I do not see any obvious impact on Fortress Preferred Stock reintroducing dividends during the 1-20 months’ timeframe. A sale of pipeline assets or a buyout of the entire company is seen as a possibility, but given today's valuation and Fortress' declining stake, it should not have a decisive impact on Fortress' decision to reinstate dividends. What will have a positive impact, relative to reintroducing dividends, is the decreasing R&D and other Operating expenses below on the Fortress consolidated balance sheet.
d. Avenue Therapeutics
Brief description: Avenue Therapeutics is a specialty pharmaceutical company focused on the development and commercialization of therapies for the treatment of neurologic diseases. The Company owns a diverse portfolio that includes first-in-class programs in a high-value neurologic landscape with significant unmet patient need.
Ownership: 9.2%
Annual Equity Dividend: 2.5%
Royalty: 4.5%
Pipeline: BAER-101 & IV Tramadol
Comments: Same as Mustang Bio's comments above, low value and mostly paused activity due to medical and financial reasons. Read about former asset AJ201 under “Subsidiaries Deals” below.
5. Private Subsidiaries
a. Cyprium Therapeutics
Brief description: Cyprium Therapeutics is a rare disease company with a focus on the development and commercialization of novel therapies for Menkes disease, a rare and fatal pediatric disease in copper metabolism.
Ownership: 73.1%%
Annual Equity Dividend: 2.5%
Royalty: 4.5%
Pipeline: AAV-ATP7A (Gene Therapy: CUTX-101 2.0) for Menkes (read about CUTX-101 under “Subsidiaries Deals” below)
Comments: Given the CUTX-101 deal, I think it is both right and gratifying that Cyprium's AAV-ATP7A Gene Therapy for Menkes Disease has both been moved up and given more space in the latest investor presentations. If these results (picture) from preclinical studies compared to CUTX-101 can be followed up in the clinical study, we are talking potential peak sales north of $1B (Fortress' estimated ceiling for CUTX-101) per year.
Quote and text from Delveinsights Menkes Outlook (more about them later):
“Gene therapy is being explored as a potential approach to treat Menkes disease, although targeting all affected cells poses significant challenges. Targeted gene therapy for the brain may offer a promising avenue for future research.”
b. Urica Therapeutics
Brief description: Urica Therapeutics is a clinical-stage biopharmaceutical company developing a novel drug to address the significant unmet needs for the treatment of Gout and other conditions associated with Hyperuricemia.
Ownership: 69.6%
Annual Equity Dividend: 2.5%
Comments: Read under “Subsidiaries deals” below about Dotinurad.
c. Helocyte
Brief description: Helocyte is developing a novel immunotherapy Triplex for the prevention and treatment of Cytomegalovirus (CMV).
Ownership: 83%
Annual Equity Dividend: 2.5%
Royalty: 4.5%
Pipeline: Triplex
Fortress estimated potential peak global sales per year:
Solid Organ Transplant - $1B+
Hematopoietic Stem Cell Transplant - $500M+
Non-Hodgkin's lymphoma (NHL) - $1B+
HIV - $10B+
Comments: Given previous analysts on peer HOOKIPA’s HB-101 (before the failure) and comparative study results against Mercks PREVYMIS and its sales (2024: $785M, +33%), Helocyte's market value should, according to me, already today be significantly higher than what the entire Fortress is priced at.
Together with Dotinurad, Triplex is the most promising asset in the pipeline/portfolio according to me and Fortress CEO (statement from an Investor Conference 2024). However, as this analysis applies to Fortress Biotech Preferred Stock and the theory of reintroducing dividends in 1-20 months, Triplex will not come into play for commercialization before then. I believe, given Triplex's previous study results, and all the economic contributions it continues to make, that Fortress want to keep Triplex in their own hands for as long as possible despite the numerous ongoing studies. Especially if my title and thesis for this Preferred Stock analysis are correct, that money is starting to flowing into Fortress and its subsidiaries at the same time as the total costs are rapidly decreasing. Those of you who have followed my texts/posts in other context before knows that I have written meters of columns about Triplex already, but this will have to be saved for another time. However, I would not rule out a buyout of Helocyte in the next 1-20 months, but I don’t think I need to comment on the financial significance it would have for both Fortress Common and Preferred Stock in both the short and long term.
I think Triplex has good potential to become a "Blockbuster" for Fortress with at least $1 Billion in sales per year. This is based on the closest peer/competitor study results and current sales in relation to Triplex study results to date.
d. Oncogenuity
Brief description: Oncogenuity is developing novel oligonucleotides for the treatment of genetically driven diseases, including cancers and coronaviruses by targeting gene-silencing at the DNA level.
Ownership: 73.5%
Annual Equity Dividend: 2.5%
Royalty: 2.5%
Portfolio: ONCOlogues (Oligonucleotide Platform)
Comments: In addition to the description in the latest Annual Report (below) and the fact that they have modernized their website, activity in terms of figures in the latest Annual Report appears to remain low. It is not considered to have any impact on Fortress Preferred Stock opportunities over the reinstated dividend in the next 1-20 months.
“Oncogenuity is developing a delivery platform that allows peptic nucleic acids to enter a cell membrane and nucleus,displace the targeted mutant DNA strand, and prevent mutant mRNA transcription. Oncogenuity is seeking to optimize lead candidates targeting genetically driven cancers, including KRAS G12D, and other genetic disorders.” - P.17, Annual Report 2024
e. Cellvation
Brief description: Cellvation is a clinical-stage biopharmaceutical company developing novel cellular therapeutics for the treatment of traumatic brain injury (TBI).
Ownership: 79.2%
Annual Equity Dividend: 2.5%
Royalty: 2.5%
Pipeline: CEVA-D, CEVA-102
Comments: In addition to the description in the latest Annual Report (below) and the fact that they have modernized their investor presentation continuously for the past year (available on Fortress' website under "Presentations"), activity in terms of figures in the latest Annual Report appears to remain low. It is not considered to have any impact on Fortress Preferred Stock opportunities over the reinstated dividend in the next 1-20 months.
“Through our subsidiary Cellvation, we are developing CEVA-D, a novel bioreactor device that is designed to enhance the anti-inflammatory potency of bone marrow-derived cells without genetic manipulation, using wall shear stress to suppress tumor necrosis factor-a (“TNF-a”) production by activated immune cells. CEVA-102 is the first cell product produced by CEVA-D, and may be applicable for various indications, including the treatment of severe traumatic brain injury.” - P.17, Annual Report 2024
6. Subsidiaries Deals
a. Checkpoint Therapeutics
Ownership: 8.3%
Partner: Sun Pharmaceuticals
Status: Pending sale
Upfront payment: $28M
CVR per share:
$0.20 - $1.384M
$0.45 - $3.115M
$0.70 - $4.846M
Royalties: 2.5%
The figures above apply to Fortress' share/part in the entire deal (Source).
Comments: Fortress and Checkpoint have stated that they expect the deal to close in Q2. On May 28th, there will be an extraordinary shareholders meeting where shareholders will have the opportunity to vote for or against the deal.
Although 2.5% royalties apply to the entire pipeline of four more drugs besides UNLOXCYT, and that this could generate further revenue for Fortress given UNLOXCYT's expected beneficial combined medical opportunities, the focus for the reinstated dividend in the near term, as stated in the pitch, is solely on UNLOXCYT sales in the US and possible CVR:
“Cosibelimab shows significant promise as a treatment for advanced CSCC, offering anovel immunotherapeutic approach for patients with limited treatment alternatives. Itsdual mechanism of action, which includes PD-L1 inhibition and potential ADCC, positionscosibelimab as a unique candidate in the ICI landscape. Clinical trials have demonstratedits efficacy, with ORRs comparable to existing therapies such as pembrolizumab andcemiplimab. Additionally, its favorable safety profile, characterized by a lower incidence ofsevere irAEs, further supports its viability as a treatment option.” - (Idris, O.A.; Westgate, D.; Saadaie Jahromi, B.; Shebrain, A.; Zhang,T.; Ashour,H.M. PD-L1 Inhibitor Cosibelimab for Cutaneous Squamous Cell Carcinoma: Comprehensive Evaluation of Efficacy, Mechanism, and Clinical Trial Insights. Biomedicines 2025, 13, 889. https:// doi.org/10.3390/biomedicines13040889)
According to Sun Pharma's latest investor presentation (February 2025), they are a global company with over 43,000 employees, operating in 100 countries, of which approximately 80 of them in large-scale. With them, I believe that a potential Best-in-Class drug, if it only stops at cSCC (which I don't think it will), will sell quite well, even if it only stops at the US (which I don't think it will). Fortress Potential peak global sales for just CSCC was $300M-$500M. Over time, it will be added with a CVR and royalties on a continuous and increasing basis for years into Fortress pockets.
If we look at the cost savings on Fortress' balance sheet starting in H2 this year, with 1.5 years left until the maximum dividend cap for reinstated Preferred Stock (according to my theory), they would be significant:
A much more comprehensive text would have been in order if it weren't for the fact that the deal is in its final stages. In a possible later text for Fortress Common Share, I will be able to develop the history and potential of UNLOXCYT and the other pipeline assets in Sun Pharma's (hopefully) hands for more possible royalties in the future.
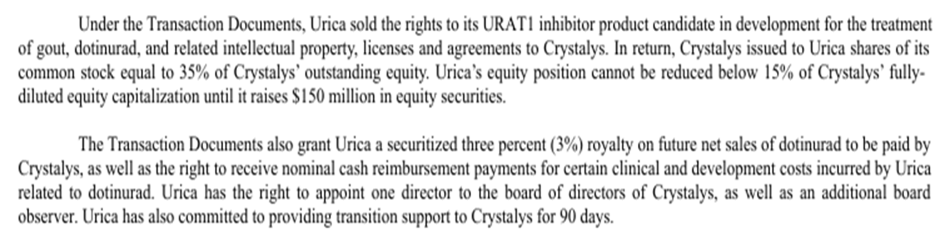
b. Urica Therapeutics
Asset deal: Dotinurad
Partner: Crystalys Therapeutics
Deal: Urica owns 35% of Crystalys and 3% royalty on future net sales of Dotinurad
Fortress estimated potential peak global sales per year: $1B+
Urica Therapeutics – Crystalys Therapeutics deal (2024-07-15)
"The APA also gives Urica the right, but not the obligation, to repurchase the sold assets for a repurchase price not to exceed $6.4 million plus accrued interest; in the event that Crystalys has not consummated a qualified financing of at least $120 million before January 8, 2026." – P.139, Fortress Biotech Annual Report 2024
Dotinurad – Initial Market
Gout
“In the United States, the prevalence of gout has risen dramatically over the past few decades, affecting approximately 3.9% of the adult population, or about 9.2 million people-” (Murdoch, R. et al. Gout, Hyperuricaemia and crystal-associated disease network common language definition of gout, 2021)
“In 2020, 55.8 million (95% uncertainty interval 44.4–69.8) people globally had gout, …”
“The total number of prevalent cases of gout is estimated to reach 95.8 million (81.1–116) in 2050, …”
Hyperuricemia
“… , hyperuricemia affects about 20% of the general populations.”
(Chen-Xu, M., Yokose, C., Rai, S. K., Pillinger, M. H. & Choi, H. K. Contemporary prevalence of gout and hyperuricemia in the United States and decadal trends: The national health and nutrition examination survey, 2007-2016. 2019).
“The global prevalence rate has been reported to be ranging from 2.6% to 36% in different populations”
(Dehlin, M., Jacobsson, L. & Roddy, E. Global epidemiology of gout: prevalence, incidence, treatment patterns and risk factors. 2020)
“The U.S. National Health and Nutrition Examination Survey (NHANES) indicates that approximately 21% of adults, or 43 million individuals, have been diagnosed with hyperuricemia.”
(Zhu, Y., Pandya, B. J. & Choi, H. K. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007-2008. 2011).
Comments: Together with Helocytes Triplex, Dotinurad is the most promising asset in the pipeline/portfolio according to me and Fortress CEO (statement from an Investor Conference 2024). However, as this analysis applies to Fortress Biotech Preferred stock and the theory of reintroducing dividends in 1-20 months, Dotinurad will not come into play for commercialization before then. However, I have written hundreds of posts about Dotinurad in other contexts with a small library of saved texts and studies that I hope to return to in a longer analysis of the future of Fortress Biotech Common stock. But for a little relevance, I think Dotinurad has potential to become a "Blockbuster" for Fortress with at least $1 Billion in sales per year. This is based on the closest peer/competitor study results and current sales in relation to Dotinurad study results to date.
c. Cyprium Therapeutics
Cyprium
Ownership: 73.1%
Annual Equity Dividend: 2.5%
Deal asset: CUTX-101 (deal below)
Status: PDUFA 30/9-2025
Partner: Sentynl/Zydus
Milestones: Up to $129 million in aggregate development and sales milestones
Royalties (on every annual sale):
3.00% of net sales up to $75M
8.75% between $75M and $100M
12.50% over $100M
PRV sale: Potential ~$150M, numbers depending on last year’s PRV sales:
IPN - $158M
ACAD - $150M
PTCT - $150M
In addition to the information (image below) about Cyprium PPS, NIH is entitled to 20% of PRV sale.
Fortress estimated potential peak global sales per year (CUTX-101): $500M - $1B
Comments: Cyprium Therapeutics is a rare disease company with a focus on the development and commercialization of novel therapies for Menkes disease, a rare and fatal pediatric disease in copper metabolism which affects between 50-225 children per year in the US. They had two drugs in their pipeline. CUTX-101 (Copper Histidinate Injection) first drug targeting Menke's disease already awarded FDA granted Breakthrough Therapy, Orphan Drug, Fast Track, and Rare Pediatric Disease Designations. Sentynl (100% owned subsidiary of Zydus Lifesciences) assumed control over development of CUTX-101 in December 2023 and will develop and commercialize the drug if approved (PDUFA 30/9-2025). Track two, which Cyprium still owns/control, is 2.0 targeting Menke's disease in AAV-ATP7A Gene Therapy, which will combine CUTX-101 with gene therapy. Preclinical studies have been done and evaluated, and they have already been awarded Orphan Drug Designation from FDA here and they expect to nominate candidate for clinical development during this year 2025.
Cyprium has 100% rights for the voucher PRV worth approximately $150M (based on last 3 PRV sales). This will be awarded upon FDA approval as CUTX-101 is directed against Menke's disease, a rare pediatric disease, which designation and requirements for PRV you can read about here.
What Is Menkes Disease?
Copper is an essential mineral that our bodies use in many ways. While the body only uses a small amount of copper, even this tiny amount is required for many functions. Copper helps with metabolism, brain functioning, blood vessel and blood cell formation, wound healing and the immune system. Copper also helps to convert iron into a usable form in the body. It is naturally available in many foods and dietary supplements.
Menkes disease, also known as Menkes syndrome, is a disorder caused by a mutation of the ATP7A gene. This ATP7A gene affects how the body transports copper and maintains copper levels (Source).
The disease thus arises from disorder of copper metabolism caused by mutations in the Copper transporter ATP7A and If untreated, premature death occurs at approximately 3 years of age.
CUTX-101 is done via an injection to replenish copper histidinate. Because so few are affected by the disease, the studies for CUTX-101 have taken a long time. The FBIO CEO Rosenwald said in an interview a while ago that study results of 14-15 years are behind the results they have today. In fact, the full research behind Cyprium today dates to 1993, over 3 decades ago (Source).
CUTX-101 - Efficacy Data
- 75-79% reduction in risk of death compared to untreated Historical Control (HC-ET and HC-LT) arms
- Increase in Median OS from 1.3 years to 14.8 years in the ET cohort
- p-value <0.0001
It is almost impossible to comprehend how convincing this effect is, and what impact it can/will have on the children and their families and environment. But the results become even more compelling when you break down the results:
Early Treatment with CUTX-101
"ET subjects who completed 3 years of treatment attend(ed) school and remain active and engaged."
Late Treatment with CUTX-101
"Most surviving LT subjects resided with their families; some require respiratory and feeding support."
Untreated Historical Control:
“Median child/patient died after 16.1 months. “
CUTX-101, Safety in ET & LT:
"CUTX-101 was safe and well tolerated."
"No TEAEs were considered related to study treatment."
I initially had difficulty getting a somewhat close overview of what the competition for Cyprium looked like in the present and what it could look like in the future. It turned out that there was an explanation for that. The competition, in clinical studies, is non-existent. I was able to learn about this in the comprehensive report “Menkes Disease Market Insights, Epidemiology, and Market Forecast-2034” from 2024 by Delveinsights. Some highlights from the report in text and images:
“Without treatment, most Menkes disease patients do not survive past age three. Early diagnosis and treatment can vastly improve a child’s survival and symptoms. Once diagnosed, treatment with copper supplements should be initiated immediately, delivered by daily subcutaneous injections. However, results can vary depending on the type of copper complex used and the severity of the ATP7A mutation.”
“Currently, no FDA-approved treatments exist for Menkes disease. However, injections of copper histidinate (CuHis), a novel freeze-dried formulation, have demonstrated potential in enhancing blood copper levels and improving neurodevelopmental outcomes, although its efficacy in older symptomatic patients remains uncertain.”
“The treatment landscape is evolving, with promising candidates in the pipeline, including Cyprium Therapeutics’ /Sentynl Therapeutics’ CUTX-101 (Copper Histidinate) and Cyprium Therapeutics’ AAV-ATP7A Gene Therapy”
There was no other drug candidates mentioned in the Menkes report. So, the only competitor for Cyprium's cash inflows from milestones and royalties from Sentynl/Zydus can only come from themselves for the foreseeable future. Because remember here that the studies from CUTX-101, where we may now be months away from a first FDA approval for Menkes, date back to 1993 and are exceptionally strong. The barriers to entry are very high even for Big Pharma to even dare to try to compete.
Two Healthcare Analysts at ROTH MKM (2024-08-14), whose job is to have a certain discount for the probability of a drug approval, assessed before even Sentynl/Zydus had its NDA application approved at 94% and the same for the chance of PRV, i.e. an equality that FDA approval also gives PRV. The results speak for themselves, there are no other medical help for children who die before the age of 3 without help from CUTX-101.
CUTX-101 – Future sales
PRV
If you take the average of the 3 latest PRV sales at $152.66M and throw in a discount/safety margin of 15% lower, I calculate a PRV sale of $129.8M.
Milestones
Historically, Fortress deals linked to milestones have been structured so that they can easily achieve the full amount for milestones upon FDA approval, which in this case is $129M. A discount/safety margin of 25% lower also gives $96.75M.
Reasonably (I've searched hard but can't find details for milestones in this deal) milestones are largely built and based on regulatory approvals globally starting with the US and FDA on September 30th of this year. I believe these approvals will come quickly (read below).
Royalties
Fortress estimates for global peak sales are $500M-1B. In simple terms, the average drug usually reaches peak sales somewhere between 5-10 years depending on everything from regulatory approvals in the world, marketing, distribution, sales ability, competition, expanded indications, etc. Regardless of whether it takes more or less than 5-10 years, Cyprium's drugs (CUTX-101 & AAV-ATP7A if possible, at a later stage) will be completely alone in the market for Menkes.
When it comes to these Rare Diseases with Orphan Drug status, history clearly speaks for rapid regulatory approval for large parts of the world after the FDA gives the go-ahead. Now we should remember that CUTX-101 has been granted all the drug statuses that one can get and wants to introduce a PDUFA in:
- Breakthrough Therapy
- Orphan Drug
- Fast Track
- Rare Pediatric Disease Designations
Here are a couple of helpful texts regarding Rare Disease that has been awarded Orphan designation from the FDA and what applies in my home region of Europe and the EMA:
Orphan designation: Overview (Source)
Regulatory Processes for Rare Disease Drugs in the United States and European Union: Flexibilities and Collaborative Opportunities (2024) (Source)
Orphan incentives (Source)
FDA orphan drug designation vs. EMA orphan drug designation (Source)
It is usually said that 6-12 months in general for a Rare Disease that has received FDA approval in Europe after the FDA has approved the drug, but that it can go faster than that, among other things due to the type of disease and effect/safety that the drug is supposed to cure/alleviate. I don't know what will be on the tables of the various drug agencies around the world from H2 2025 onwards, but, I find it hard to believe that it is wise/human to prioritize the review of something else so much faster than CUTX-101 for Menkes.
Add then the knowledge that only 50-220 patients per year in the US are affected and a large proportion of those children affected in recent years are already being treated within CUTX-101 studies, and I have a very hard time seeing a more average 5-10 years to peak sales that also applies to CUTX-101. When the diagnosis is made and the "choice" is between extending life expectancy by an average of 13.5 years, and with significantly better quality of life along the way, or that the child will die months later, it is not even a choice, the order for the CUTX-101 injection from Sentynl/Zydus will come immediately.
Now read again what I wrote about what determines the time for peak sales on average 5-10 years previously above and connect it to the CUTX-101 expected reality:
Regulatory approvals – Rare disease with no other options speaks for early regulatory approvals even outside the US if FDA approves
Competition – None
Marketing – Barely needed given the two answers above
Distribution & Sales ability – Few patients and large global company behind (Zydus)
Expanded indications – No
Still, to take all the safety margins and lifebuoys that are available, let's put ourselves in the range and calculate 7 years to peak global sales and put ourselves in the lower peak ($500M-$1B) amount of $600M:
Year – Sales – Royalties
2026: $70M - $2.1M
2027: $180M – $22.5M
2028: $300M - $37.5M
2029: $390M - $48.75M
2030: $480M - $60M
2031: $560M - $70M
2032: $600M - $75M
Royalties: $315.85M
PRV: $129.8M
Milestones: $96.75M
Royalties to peak global sales: $315.85M
Total: $542.4M
Note that without all the safety margins/discounts, the sum could easily have added a couple of hundred million, and above all much earlier as I believe peak global sales will be closer to 4 years than my exemplified 7 years. Note that this is also only up to peak and that it is currently somewhat impossible based on the current state of research that anyone without Cyprium themselves (CUTX-101 2.0) can compete at the time of year 2032 that I refer to (remember previous references about decades of research). And of course sales and royalties won't stop just because they reach a plateau, they will continue to flow in.
If you want to calculate the net, add NIH's 20% right of PRV and possible outcome for Cyprium PPS based on:
Short Summary: There are currently no approved drugs for Menkes and the only competition in clinical trials will come from themselves for the foreseeable future. CUTX-101 studies have been ongoing for three decades, so understandably the market to entry is incredibly tough and the competition is understandably weak thereafter. Healthcare analysts' (ROTH MKM, 14/8-2024) chances of FDA approval and PRV at 94% for CUTX-101 even before NDA accepted now in January.
d. Caelum Biosciences
Caelum
Ownership: 42% (sold)
Asset deal: CAEL-101
Partner: Alexion/AstraZeneca
FDA approval: $19.6M
Milestones (payment at latest 31/12 the year milestones are reached):
$10.5M when sales exceed $250M in total sales
$21M when sales exceed $500M in total sales
$31.5M when sales exceed $750M in total sales
$42M when sales exceed $1B in total sales
Total potential: $124.6M
Estimated potential peak global sales per year: ~$1B
Comments: AL amyloidosis is a rare, severe, progressive, systemic disease caused by free light chain misfolds due to plasma cell dyscrasia into amyloid fibrils. Amyloid fibrils deposit into organs, leading to severe damage and dysfunction and death.
Anti-plasma cell dyscrasia, which suppresses plasma cell growth, is a standard-of-care (SOC) treatment. However, there are no FDA-approved treatments that target fibrils that have already been deposited in organs.
CAEL-101 is a chimeric monoclonal antibody designed to bind a cryptic epitope on misfolded kappa and gamma light chains and resulting amyloid fibrils, leading to their removal from organs and tissues.
Extended Treatment With CAEL-101 Plus SOC Therapy Proves Safe in Light-Chain Amyloidosis:
“The addition of the chimeric monoclonal antibody CAEL-101 to standard therapy with cyclophosphamide, bortezomib (Velcade), and dexamethasone with or without daratumumab (Darzalex) demonstrated a manageable toxicity profile with prolonged clinical benefit in patients with amyloid light-chain amyloidosis, according to findings from a phase 2 trial (NCT04304144) that were presented at the 2023 EHA Congress.
Responders were defined as having at least 30% reduction from baseline and decrease of NT-proBNP above 300 ng/L in patients with a baseline NT-proBNP of at least 650 ng/L. Stable disease was defined as neither response nor progression, the latter of which was defined as having at least 30% increase from baseline and at least 300 ng/L of NT-proBNP without decline in eGFR at least 25% from baseline.
‘Among patients with AL amyloidosis treated with CAEL-101 for 18 months, CAEL-101 was generally well tolerated without evidence of toxicity in patients with AL amyloidosis. Most treatment-emergent adverse effects were mild to moderate, and cardiac response persisted even after cessation of anti–plasma cell dyscrasia treatment,’ lead study author Michaela Liedtke, MD, associate professor of medicine (hematology) at Stanford Medicine in California, said in a presentation of the data.” - https://www.onclive.com/view/extended-treatment-with-cael-101-plus-soc-therapy-proves-safe-in-light-chain-amyloidosis
There are two ongoing global Phase 3 studies of CAEL-101 for Stage IIIa and Stage IIIb AL amyloidosis (ClinicalTrials.gov identifiers: NCT04512235 and NCT04504825). The P3a patient part should have been completed by April 7 and P3b is expected to be completed this week on May 7. Then a couple of months of collecting and compiling the results await. Alexion has active job applications in the US and Japan for Regional Managers with a job description that is based solely on the drug CAEL-101, so if I may guess, Alexion/AstraZeneca assumes that CAEL-101 will be approved.
AstraZeneca Q1 (2025-04-29) - Update on CAEL-101
Alexion (AstraZeneca's Rare Disease Division) continues to confirm the readout for P3a-b during H2 this year. My guess is that, if the timeline continues to hold, the goal, like previous presentations in Al amyloidosis, is to present the data at the ASH Annual Meeting which will be held in 2025, December 6-9.
Short Summary: Two healthcare at ROTH MKM analysts rated the chance of FDA approval at 72% in Q3 2024 and since then new positive data has been presented (ASH Annual Meeting, December 2024). With positive P3 results, I expect PDUFA sometime in Q2-Q3 next year 2026, given that CAEL-101 has both Fast Track and Orphan designations in both the US and EU, which makes Fortress' $19.6M directly upon FDA approval really come into play for my timing aspect for reinstated dividends in Fortress Preferred Stock within 1-20 months. Milestones are structured in such a way that it is my belief that all $124.6M will accrue to Fortress in the future with FDA approval. The milestones for sales from CAEL-101 will be a longer bet on safer dividends in the stock and an increase in Fortress Common Stock.
Also worth noting Fortress bought CAEL-101 for about $1.5M back in the day and has already collected $56.9M in milestones in the deal.
e. AEVITAS
Brief description: Aevitas Therapeutics - Sold to 4D Molecular Therapeutics in April 2023 for up to ~$140M in future milestone proceeds, plus royalties from AVTS-001 (AAV.SFH Gene Therapy), now 4D-175 for Geographic Atrophy:
“Aevitas will receive the payment as potential late-stage development, regulatory and sales milestones. The firm will also receive single-digit royalties on net sales.” (Source)
From Q4 2024 (28/2-2025):
“Paused significant additional capital allocation and investment, pending additional financing or partnerships for the following therapeutics:
4D-175 for geographic atrophy
4D-725 for alpha-1 antitrypsin deficiency lung disease
4D-310 for Fabry disease cardiomyopathy”
Comments: The reprioritization/pause for 4D-175 was a shame given the promising reporting that initially came from 4D Molecular, but, understandable given their economic situation and the need to try to get the 4D-150 project done first. Hopefully, P1 for 4D-175 can start after that. As milestones and royalties are negotiated based on a late stage in development and sales, it would not have affected Fortress' possible need to reinstate the dividend within 1-20 months.
f. Avenue Therapeutics
Last week there was a press release about the settlement and resale of AJ201, which could lead to future income in the long term and savings on further study costs in the short term, this because AJ201 was really the only active drug in the pipeline that was in ongoing clinical trials.
7. Financial
Reporting in an Investment Company like Fortress Biotech
When an investment company reports its financial results according to applicable accounting principles (such as IFRS here in Sweden or equivalent local standards), the aim is to provide a comprehensive and holistic view of the financial position of the group. Here are some key reasons:
1. Consolidated perspective: An investment company that owns subsidiaries is considered as a single entity where the parent company's reporting is supplemented by the subsidiary's figures. By consolidating the income, expenses, assets and liabilities of all subsidiaries, a true and fair view of the entire business is obtained, rather than just seeing an isolated part of it.
2. Transparency for investors and stakeholders: Complete reporting enables investors, analysts and other stakeholders to assess the group's performance and risk profile. This is important for making informed investment and business decisions, as the total exposure – both positive and negative – is clearly visible.
3. Comparability and consistency: By reporting all income and liabilities in a consolidated manner, it becomes easier to compare the investment company's performance with other companies and industry standards. This provides a unified picture that is necessary to analyze overall profitability and financial stability.
4. Regulatory requirements: Accounting regulations often stipulate that the results of subsidiaries should be consolidated if the parent company exercises control over them. This ensures that no material information is omitted, which in turn strengthens the reliability of reporting and accounting integrity.
By reporting all income and liabilities – regardless of whether they belong to the parent company or one of the subsidiaries – it becomes clear how different parts of the group contribute to the overall financial picture, which increases both transparency and the ability to make well-balanced analyses.
a. Revenue
Comments: $55.1M of $57.675M from Journey Medical portfolio sales, $1.5M relates to collaboration revenue from Sentynl for the NDA submission acceptance milestone relating to CUTX-101, and $1.0 million in other revenue relates to a milestone payment from Cutia related to the approval of AMZEEQ (also Journey Medical) in China.
b. Operating Expenses
Research & Development
As I tried to point out under headings 3–5 above, R&D will decrease significantly for Avenue, Checkpoint, Journey Medical and Mustang. The short version:
Avenue – Sold back AJ201 which was the only drug in active clinical studies.
Checkpoint – UNLOXCYT was granted FDA approval on 204-12-13 and no further studies have started/continued in 2025 to my knowledge. The company will most likely be sold now in Q2 (pending deal).
Journey Medical – EMROSI was granted FDA approval on 2024-11-04 and was the only asset in the pipeline.
Mustang – More or less paused operations until at least Q4 2025 while waiting for word on funding for the studies for MB-106 and MB-109 according to communication from the company.
R&D at Fortress is inclusive of annual equity dividend income received from the subsidiaries. The total figure in Fortress (which includes the private subsidiaries) is also kept down thanks to the several contributions Helocytes Triplex has received in the multi-million class.
Intangible Assets
These texts and figures regarding Journey Medical's Intangible Assets under Fortress' financial statements, I think, explain well that the figures may go down in 2025. This is mainly because of one-offs with $3M+$15M milestones to DRL for NDA and FDA acceptance for EMROSI and $4M FDA fee at application for approval to FDA. The repayment will not be a problem, according to me, given how the SWK loan (see below) is structured and now that EMROSI's sales money will start flowing in.
Stock-based compensation
An explanation is needed for the large difference from 2023 to 2024 for Checkpoint and Journey Medical. These are almost exclusively attributed to both receiving their first FDA approvals for assets they co-developed in UNLOXCYT and EMROSI. Therefore, Journey Medical and Checkpoint (regardless of whether the sale closes in 2025 or not) will see their numbers decline significantly already this year.
Selling, General & Administration
Given the outcome for Avenue, Checkpoint and Mustang during R&D, I expect the costs to decrease here as well, especially for Checkpoint if they are getting acquired by Sun Pharma. Journey Medical's around 35 people in the Sales Team will, as I wrote above, increase continuously to 40-45 people over a couple of years as they approach peak sales for EMROSI in the US, so I don't think their numbers will change significantly based on that. However, it could increase somewhat if this item also includes marketing regarding EMROSI. Overall, the total should still be able to go down quite a bit if Checkpoints is sold.
Dotinurad
In Urica Therapeutics two segments under subsidiaries 3-5 and here under included under Fortress, I have not named the future cost savings regarding Dotinurad after the deal with Crystalys (2024-07-14). This is because I had difficulty placing it and therefore thought it deserved its own segment.
Since the patient part of Phase 1B was completed before the deal, that it is not such a substantial savings in itself. However, based on Fortress' communication, Crystalys is being prepared for two pivotal Phase 3 (almost certainly for Gout and Hyperuricemia) in the US. The financial cost of that, starting presumably in Q4 2025-Q1 2026, and that it will happen in Crystalys after the condition from Urica/Fortress that they should add at least $120M in investment by January 2026, is an opportunity cost that would have been large if it had been solely on Urica/Fortress given the financial and human resources they have today. The cost savings in real figures from 2024-2025 for this will not be so noticeable, however and again, the opportunity cost going forward starting in 2025-2026 is significant.
Those of you who have followed my texts before in other contexts also know that an investment with strong connections to Big Pharma in Novo Nordisk made an investment in Crystalys in December (more on that another time).
Others
In February 2025 Mustang announced it had concurrently exited the lease for its manufacturing facility in Worcester, Massachusetts and sold certain fixed assets including furniture and equipment to AbbVie Bioresearch Center for $1.0 million.
c. Debt
Oaktree – Fortress Biotech’s debt & SWK – Journey Medicals debt
Fortress Biotech - Oaktree
In July 2024, Fortress announced the reduction of total debt outstanding and the entry into a new $50 million term loan with Oaktree Capital Management with a maturity in 2027. The Company borrowed $35.0 million under the agreement on the closing date and can draw up to an additional $15.0 million at the lenders’ discretion to support future business development activities. In connection with the new term loan, Fortress repaid the prior $50 million 2025 term loan with Oaktree.
The Oaktree loan is structured so that they first only need to pay interest on the loan itself every quarter, then 50% of the outstanding loan by March 2027 and the remaining 50% in the period from March to July 2027. Given the expected broadly increasing revenues I referred to earlier (and will come back to) starting in 2025 and with accelerating force in 2026 and beyond, this is a very favourable arrangement for Fortress. However, it comes with certain conditions as a guarantee. One is that Journey Medical can maintain TTM sales of $50M, which they already do ($56M 2024). In addition, with EMROSI's sales start 2025-03-24, there should be no doubt at all (in my opinion) that during the period until the loan maturity in July 2027, that Journey will fall below $50M in TTM sales. In fact, there is even a note in the Oaktree document that states "due to the approval of Emrosi, the minimum net sales amount will increase by $7.5 million each quarter, beginning in the third quarter of 2025", this quote I believe refer to all the way to the loan maturing in July 2027. The second is that Fortress and can maintain liquidity of $7M. This should also not be a problem with increased revenues and lower operating expenses. Here it is also necessary to remember that in Fortress's item for cash in the balance sheet, the privately held subsidiaries are included, and to maintain the parent company and them, Fortress has always had available cash much higher than the $7M required here in liquidity.
Journey Medical - SWK
Once Journey Medical had the EMROSI data in hand, they did everything in their power to maximize shareholder value given the bright future they now saw on the horizon. As I have touched on before, it even pulled the sales staff and marketing for the existing commercial portfolio. Diluting shareholders, given how crazy low Journey Medical was trading, by printing new shares was their absolute last preference. So, in December 2023 they secured a term loan facility of $20M (increased to $25M in July 2024). They were confident to secure FDA approval for EMROSI in 2024 given the superior efficacy in head-to-head versus SoC and placebo-like side effects, and they knew then that they needed to have $18M ($3M NDA acceptance + $15M FDA acceptance) available for milestones for Dr. Reddy Laboratories plus $4M for FDA fee application for approval.
Like Fortress Oaktree, it also needs to be noted what favourable conditions prevail with the back-heavy setup:
“Beginning in February 2026, Journey is required to repay a portion of the outstanding principal of the Term Loans quarterly in an amount equal to 7.5% of the principal amount of funded Term Loans, with any remaining principal balance due on the maturity date. If the total revenue of Journey, measured on a trailing twelve-month basis, is greater than $70.0 million as of December 31, 2025, the principal repayment start date is extended from February 2026 to February 2027, at which point Journey is required to repay a portion of the outstanding principal of the Term Loans quarterly in an amount equal to 15% of the principal amount of funded Term Loans, with any remaining principal balance due on the maturity date.” – Fortress Biotech Annual Report 2024, P.149
Note: Then there are small loans in some of the subsidiaries with less relevance, for them I refer to the reports from Fortress.
d. Dividend
First some general important info in about the Fortress Preferred Stock, read the SEC document:
Fortress Biotech – Preferred Stock History & Structure
Certificate of Designations of Rights and Preferences
9.375% Series A
Cumulative Redeemable Perpetual Preferred Stock
I also find these two paragraphs from P.37 in the latest Annual Report (2024) extremely important to read as they address the conditions for reinstated dividends and that Fortress is not allowed to use S-3, which applies until the day the dividend is reinstated:
Regarding “… ability to pay cash dividends on our Series A Preferred Stock in the future requires us to have either net profits or positive net assets (total assets less total liabilities) over our capital, and that we have sufficient working capital in order to be able to pay our debts as they become due in the usual course of business.”, here is the latest Consolidated Balance Sheet:
Dividend “Dividends on Series A Preferred Stock accrue daily and will be cumulative from, and including, the date of original issue and shall be payable monthly, at the rate of 9.375% per annum of its liquidation preference, which is equivalent to $2.34375 per annum per share. The first dividend on Series A Preferred Stock was payable on December 31, 2017 (in the amount of $0.299479 per share) to the holders of record of the Series A Preferred Stock at the close of business on December 15, 2017.” – Annual Report 2024
The last dividend was paid in June 2024. This means that the accumulated dividends today consist of:
2024
July – 1 x $0,1953 = $0,1953 Dividend per share
August - 2 x $0,1953 = $0,3906 Dividend per share
September - 3 x $0,1953 = $0,5859 Dividend per share
October - 4 x $0,1953 = $0,7812 Dividend per share
November - 5 x $0,1953 = $0,9765 Dividend per share
December - 6 x $0,1953 = $1,1718 Dividend per share
2025
January - 7 x $0,1953 = $1,3671 Dividend per share
February - 8 x $0,1953 = $1,5624 Dividend per share
Mars - 9 x $0,1953 = $1,7577 Dividend per share
April - 10 x $0,1953 = $1,9530 Dividend per share
Based on today’s share price of $6.61, the cumulative dividend yield today (2025-05-05 )is 29.50%.
Future remaining dividends within the 1–20-month time frame I refer to under "Quick Pitch" above:
May - 11 × $0,1953 = $2,1483 Dividend per share
June - 12 × $0,1953 = $2,3436 Dividend per share
Juli - 13 × $0,1953 = $2,5389 Dividend per share
August - 14 × $0,1953 = $2,7342 Dividend per share
September - 15 × $0,1953 = $2,9295 Dividend per share
October – 16 × $0,1953 = $3,1248 Dividend per share
November - 17 × $0,1953 = $3,3201 Dividend per share
December - 18 × $0,1953 = $3,5154 Dividend per share
Based on today’s share price of $6.61, the cumulative dividend yield at year-end would be 53.17%.
2026
January - 19 × $0,1953 = $3,7107 Dividend per share
February - 20 × $0,1953 = $3,9060 Dividend per share
Mars - 21 × $0,1953 = $4,1013 Dividend per share
April - 22 × $0,1953 = $4,2966 Dividend per share
May - 23 × $0,1953 = $4,4919 Dividend per share
June - 24 × $0,1953 = $4,6872 Dividend per share
July - 25 × $0,1953 = $4,8825 Dividend per share
August - 26 × $0,1953 = $5,0778 Dividend per share
September - 27 × $0,1953 = $5,2731 Dividend per share
October - 28 × $0,1953 = $5,4684 Dividend per share
November - 29 × $0,1953 = $5,6637 Dividend per share
December - 30 × $0,1953 = $5,8590 Dividend per share
Based on today's share price of $6.61, the cumulative dividend yield at the next year-end in 2026 would be 88.64%.
There are currently 3,427,138 shares of Fortress Preferred outstanding. With the 10 paused and thus accumulated dividends, this means that Fortress has a dividend liability of $6.69M and that a monthly dividend therefore costs them $0.669M. Which means that if Fortress Biotech Preferred Stock reinstates its dividend and keeps it intact sometime between now and the end of 2026, you will receive $5.8590 in dividends for every share you own priced at $6.61 today, if you hold the stock until mid-December 2026 (less than 20 months). A dividend yield of 88.64%, at today's share price.
e. Future Expected Revenues (2025-2026)
Fortress & their Private Subsidiaries:
Checkpoint
Upfront payment from Sun Pharma deal:
$28M
CVR:
$0.45 - $3.115M
Cyprium
PRV:
$152.66M last 3 average PRV sales with a discount/safety marign of 15%: $129.8M ->
$129.8M-$25.96M (NIH 20% rights)-$16M (Cyprium PPS) = $87.84M
Milestones:
$129M with a discount/safety margin of 25%: $96.75M ->
75% of those $96.75 milestones due for a presumably high share at the FDA and global approvals thereafter under 2025-2026 = $72.56M
Royalty:
$70M in sales 2026 (3.00% of net sales up to $75M) = $2.1M
Caelum deal (sold)
CAEL-FDA approval: $19.6M
Fortress & their Private Subsidiaries - Consolidate Net Revenue
2024 Revenue: $1.5M
2025–2026 Expected Revenue: $213.22M (or $106.61M in average per year)
Comments: I hope it is clear that I have included significant discounts/margins in my calculations. I have also not included a single dollar of the 2.5% royalties from Sun's sales of UNLOXCYT which should be out on the market for at least a year if the deal closes now in Q2. With full CVR from Checkpoint and a CUTX-101 PRV sale close to $150M would contribute to around $231M.
Journey Medical
ROTH MKM has been spot-on in predicting Journey's sales for several quarters in a row but missed the mark recently in Q4 given too high expectations for ACCUTANE. Here are their expectations for EMROSI alone for 2025-2026:
2025
Q1: $0M
Q2: $5M
Q3: $14M
Q4: $21M
Total: $40M
2026
Total: $80M
I choose to take this $120M and apply a safety margin/discount of 15%, resulting in $102M to be added to the remaining expected sales from the commercial portfolio. Journey is required to pay royalties to DRL ranging from approximately 10%-14% on net sales of EMROSI (subject to certain possible reductions). As these royalties may look similar as in other Fortress deals (read above), the percentage tends to be in the lower range initially and increase to the higher range closer to expected peak sales. I therefore choose to calculate 11% royalties to DRL for these $102M in EMROSI sales in 2025-2026, which ends up at $90.78M.
Then I choose to take their latest 2024 Revenue x $56,134M = $112.268M, before I also set a safety margin/discount of 15% there as well, meaning that the current portfolio will lose 15% in sales over the next two years, and then receive $95.43M.
2025-2026 EMROSI: $90.78M
2025-2026 Current Portfolio (only EMROSI excluded): $95.43M
2024 Revenue: $56.134M
2025–2026 Expected Revenue: $186.21M (or $93.105M in average per year)
Comments: Here too, I hope you could see that I have been cautious in my assumptions. However, it may be appropriate to be a little cautious given the increased competition ACCUTANA has started to meet in H2 2024 and the expected increased competition for QBREXZA from H1 2025. At the same time, and this is very important to note, I have not included any sales deal with upfront payment on any of Journey's commercial products including EMROSI. And, I do believe that Journey will close at least one lucrative sales deal for primarily EMROSI during 2025-2026. The reason why I have not included a possible deal in my calculations is that Journey, in its negotiating position with EMROSI, and that they will soon turn to net profit, may choose to structure the deal in different ways. When Journey closed its $19M upfront (plus up to $45M in total) deal for QBREXZA for parts of Asia, they were more in need of that upfront money based on the balance sheet then and there. Today, the situation looks different, and Journey could negotiate a low upfront payment to instead prioritize a higher royalty percentage for sales in, for example, the EU. My guess is, however, that you might want some upfront payment if you see an opportunity for an acquisition to broaden either the commercial portfolio today and/or a product in development for the pipeline in hopes of later commercialization.
Checkpoint
Note! I expect Checkpoints to be sold in Q2 as described above. If this happens, Checkpoint's revenue column will be included under "Fortress & Private Subsidiaries" as its $28M + CVR figure example only applies to Fortress' share in the Sun Pharma buyout.
Avenue & Mustang
2024 Revenue: $0
2025–2026 Expected Revenue: $0
Comments: I wouldn't be surprised if all of Avenue and Mustang or parts of the pipelines are sold in 2025-2026, but I think it's too aggressive to count on such a scenario, especially given their market value and Fortress' low ownership percentage today after several forced new issues.
f. Future Expected Savings (2025-2026)
Here I will report and summarize the savings I see possible over 2025-2026 for the entire group, based on 2024 figures for “Total Operating Expenses”.
Fortress & their Private Subsidiaries
Total Operating Expanses
2024: $14.5M
2025–2026: $29M ($14.5M x 2)
2025-2026 - Expected savings: ($6M)
Comments: Based mostly on savings in Urica after the Dotinurad deal with Crystalys but increased costs for reinstated dividend, expected P1 start for AAV-ATP7A (CUTX-101) and Helocyte increasingly more and larger studies for Triplex (if it happens without a partner).
Journey Medical
Total Operating Expanses
2024: $69.8M
2025–2026: $139.6M ($69.8M x 2)
2025–2026 - Expected savings: $15M
Comments: $22M on-offs that Journey booked in 2024 as milestones to DRL and FDA fee will disappear plus they don't have to start repaying the SWK loan until February 2026 at the earliest. In addition, a larger item that has been on the development cost of EMROSI will disappear. However, expect higher personnel and marketing costs considering EMROSI.
Checkpoint
Total Operating Expanses
2024: $56.22M
2025–2026: $112.44M ($56.22M x 2)
2025–2026 - Expected savings: $90M
Comments: This is based on Sun Pharma's purchase of Checkpoint going through now in Q2 2025.
Avenue
Total Operating Expanses
2024: $11.28M
2025–2026: $22,56M ($11.28M x 2)
2025–2026 - Expected savings: $1M
Comments: Hard to imagine in this case. Licensing back AJ201 will save several million over two years, at the same time it is probably not out of the question that they will continue with BAER-101 in a clinical trial if they manage to secure funding.
Mustang
Total Operating Expanses
2024: $16.25M
2025–2026: $32.50M ($16.58M x 2)
2025–2026 - Expected savings: $1M
Comments: Again, hard to guess. In 2025, a lease has already been sold and they will be able to save a lot on the wait I described for MB-106 and $MB-109 before Q4. The possible contributions can hopefully keep costs down somewhat in a more active 2026.
g. Financial Summary
Fortress & their Private Subsidiaries
Total Net Revenue
2024 Revenue: $1.5M
2025–2026: $3M ($1.5M x 2)
2025–2026 Expected Revenue: $213.22M (or $106.61M in average per year)
Total Operating Expanses
2024: $14.5M
2025–2026: $29M ($14.5M x 2)
2025-2026 - Expected savings: ($6M)
Total
$213.22M - $6M = $207.22M increase (or $103.61M per year)
Journey Medical
2024 Revenue: $56.13M
2025-2026: $112.26M ($56.13M x 2)
2025–2026 Expected Revenue: $186.21M (or $93.105M in average per year)
Total Operating Expanses
2024: $69.8M
2025–2026: $139.6M ($69.8 x 2)
2025-2026 - Expected savings: $15M
Total
$186.21M + $15M = $201.21M increase (or $100.605M in average per year)
Checkpoint
Total Operating Expanses
2024: $56.22M
2025–2026: $112.44M ($56.22M x 2)
2025-2026 - Expected savings: $90M
Total
$90M increase (or $45M in average per year)
Avenue
2024 Revenue: $0
2025-2026: $0
2025–2026 Expected Revenue: $0
Total Operating Expanses
2024: $11.28M
2025–2026: $22,56M ($11.28M x 2)
2025-2026 - Expected savings: $1M
Total
$1M increase (or $0.5M in average per year)
Mustang
2024 Revenue: $0
2025-2026: $0
2025–2026 Expected Revenue: $0
Total Operating Expanses
2024: $16.25M
2025–2026: $32.50M ($16.58M x 2)
2025-2026 - Expected savings: $1M
Total
$1M increase (or $0.5M in average per year)
Total expected change in value over the two years 2025–2026 compared to 2024 balance sheet:
Fortress & their Private Subsidiaries: +$207.22M
Journey Medical: +$201.21M
Checkpoint: +$90M
Avenue: +$1M
Mustang: +$1M
Total: +$500.43M (or $250.215M in average per year)
Discussion: For the analyst consensus first, yes, I believe that a change in value of over half a billion on this balance sheet will mean that Fortress will reinstate a dividend for Fortress Biotech Preferred (FBIOP) with accumulated dividends today at a cost of $6.69M and that a monthly dividend therefore per month $0.669M. Especially since Fortress and its Preferred Stock have continuously paid dividends for several years before the pause in July 2024, with worse financial conditions than they have today, before this more than half a billion in predicted value changes can begins to register in the reports for 2025-2026.
In a second step, I just want to mention this half a billion in relation to the scope of outcomes required to actually get there. Because despite the reported very high probability that CUTX-101 will be approved by the FDA with accompanying milestones, royalties and PRV to be able to sell, EMROSI's clinical superiority now that it reaches the market, Checkpoint's very likely buyout with the following CVR, or CAEL-101's likely chance of FDA approval, it is still a difficult-to-navigate industry in Pharma/Biotech. More about that will of course be discussed under "Risks" below, but, my point here and now is that given these +$500M, I believe that there are outcomes from these that can be delayed or completely fail and the finances of Fortress Biotech in general and its future possibility of dividend will still not be under major pressure.
The third and final comment regarding these value changes that I predict during 2025-2026 must nevertheless briefly cover from 2027 onwards, although my overall analysis in this document stops before then with the prediction of a reinstatement of dividend in FBIOP within 1-20 months (i.e. by December 2026 at the latest). If you don’t remember, you need to rewind and read the outcomes that I and healthcare analysts see for assets such as EMROSI, UNLOXCYT, CUTX-101 and CAEL-101 from 2027 onwards. To exemplify:
EMROSI: Included $40M for 2025 and $80M for 2026. Expected to sell for $200M for FY2028 and should then be at least $100M away from peak global sales per year, with right partner/partners outside US, based on ORACEA sales history.
UNLOXCYT: Not including a single dollar from the 2.5% royalties when Fortress estimated peak global sales in cSSC alone at $300M-$500M. "Alone” in relation to the two competitors/peers for cSCC in Immune Checkpoint Inhibitors (anti-PD-L1 or anti-PD-1) in Keytruda and Libtayo which together had sales of $30.72B in 2024, across a wide spectrum of approved indications and combinations. With UNLOXCYT's "dual mechanism" Checkpoint has long argued that UNLOXCYT combination opportunities look very promising both within its own pipeline (mainly Olafertinib), but also within this (UNLOXCYT brand name for Cosibelimab):
Since last year, the first collaborations for a combination with partners have also been underway (Source).
CUTX-101 Milestones: Opportunity for $56.44M more
CUTX-101 Royalties: Only include $2.1M in my conservative (remember, no competition for CUTX-101 in human clinical studies today) path to peak global sales:
Year – Sales – Royalties
2026: $70M - $2.1M
2027: $180M – $22.5M
2028: $300M - $37.5M
2029: $390M - $48.75M
2030: $480M - $60M
2031: $560M - $70M
2032: $600M - $75M
Royalties: $315.85M total to peak (and of course not stopping just because it reaches peak).
CAEL-101: Not a single milestone of the $124.6M possible is included
Milestones (payment at latest 31/12 the year milestones are reached):
$10.5M when sales exceed $250M in total sales
$21M when sales exceed $500M in total sales
$31.5M when sales exceed $750M in total sales
$42M when sales exceed $1B in total sales
Which should have good possibilities with Alexion/AstraZeneca who will be responsible for the commercialization, in an indication where there is currently no FDA approved drug and estimated peak global sales are ~$1B per year.
Best of all, it doesn't include Dotinurad or Triplex, which Fortress themselves believe are the assets in their entire portfolio/pipeline that they have the highest expectations for/hold the greatest value for, which I certainly agree with (but as I said before, more about them another day).
Review to see what a significant change in value can contribute to the current financial situation from last reported quarter in Q4 2024:
Here is the consensus of the few research firms that cover the company (note that this applies to Fortress Biotech Common Share FBIO):
8. Closest comparable peers/stocks
a. Milestones & Royalty-based Companies
Royalty Pharma - $RPRX – Market Cap: $18.63B
World’s largest buyer of biopharmaceutical royalties and a leading funder of innovation in Life Sciences. Founded 1996 and is today a global company with headquarters in the US.
A substantial company with a wide global reach and diversification of pharmaceuticals. Have historically been skilled at building that "Snowball effect" of continuous and growing cash flows that Fortress still strives for. Shares royalties as a source of revenue with Fortress but with significant differences. Royalty Pharma acquires future expected royalties while Fortress creates incentives for future royalties through founder agreements for the subsidiaries it itself establishes, manages and develops. Also lacks a main owner and has low insider ownership, unlike Fortress.
Ligand Pharmaceuticals - $LGND – Market Cap: $2.14B
Founded in 1995 and headquartered in the US. Ligand is a biopharmaceutical company focused on developing and acquiring technologies that aid in creating medicine. The company has partnerships and license agreements with various pharmaceutical and biotechnology companies. Ligand's business model is based on drug discovery, early-stage drug development, product reformulation, and partnerships. The company's revenue consists of three primary elements: royalties from commercialized products, license and milestone payments, and sale of its trademarked Captisol material.
From the outside, just with a quick look, I find them very similar to Royalty Pharma, although at an earlier stage.
DRI Healthcare Trust Units - $DHT.UN -Market Cap: $455M
DRI Healthcare Trust is a Canada-based company founded 1989 and is engaged in providing financing to advance innovation in the Life Sciences industry. DRI is involved in pharmaceutical royalty monetization and provides capital to inventors, academic institutions and biopharma companies.
Read up on Trust structures in Canada and their Pass-through taxation and focus on dividends to understand the structure of the company and the share class. Otherwise, similar to the royalty structure above, although significantly smaller than its peers above.
An interesting point of connection with Fortress is that in 2021 they bought royalties from Journey Medicals main competitor for EMROSI in ORACEA for Rosacea. This was just 3 months after Journey Medical and Dr. Reddy’s Laboratories confirmed a deal for DFD-29 (now brand name EMROSI) after already having superior study results in P2 in Germany compared to ORACEA.
(Image from DRI’s latest Investor Presentation)
XOMA Royalty - $XOMA – Market Cap: $289M
Founded in 1981 in the US and changed to its current business strategy in 2017. XOMA Royalty is a biotechnology royalty aggregator playing a role in helping biotech companies achieve their goal of improving human health. XOMA acquires the potential future economics associated with pre-commercial therapeutic candidates that have been licensed to pharmaceuticals or biotechnology companies. XOMA acquires future economic rights, the seller receives non-dilutive, non-recourse funding that can use to advance their internal drug candidate(s) or for general corporate purposes. Geographically, the company operates in Switzerland, United States, Asia Pacific, Europe and Others.
I think this image is best suited to explain the difference with XOMA against the above royalty companies in the sector:
Similar to Fortress in that they have a smaller management team and that they have listed preferred share(s).
b. Investment Companies in part-ownership
Linc - $LINC.ST – Market Cap: $403M
Linc is a company that invests in product-oriented companies within the Nordic Life Science industry. The company invests in and develops primarily small and medium-sized Life Science companies within Medical Technology and Pharmaceuticals. The investments are made in both private and public companies and in research and operating companies. Linc was founded in 1991 in Sweden where it is still active with a number of companies that already have or are striving for global expansion.
Co-owns significantly more companies than Fortress and lacks the incentives for voting control (in most holdings) and Annual Equity Dividend and Royalties from the companies. However, there are similarities with a strong Founder who in turn controls the votes in Linc with his approximately 57% of both ownership and votes.
Flerie - $FLERIE.ST – Market Cap: $331M
Flerie was founded in 2011 in Sweden and are an active long-term life science investor with a focus on Biotechnology and Pharmaceutical investments globally. The company is based in Stockholm and London and manages a portfolio of investments in Europe, Israel and the USA. The main focus is to provide organizations that primarily operate in pharmaceutical development with services and resources.
The same further description of similarities and differences compared to Lincs structure, founders and diversification also applies between Flerie and Fortress. The only spontaneous tangible difference between Linc and Flerie is that Linc has more medical technology in its portfolio while Flerie has almost only Pharma & Biotech in its portfolio.
Karolinska Development - $KDEV.ST – Market Cap: $25M
Karolinska Development was founded in 2003 in Sweden where it still has its roots operating in the investment industry and focuses on long-term equity investments in biotechnology and pharmaceutical companies. The company's goal is to develop promising medical innovations stemming from academic research from Karolinska Institutet and other universities in the Nordic region. The company's portfolio includes companies in sectors such as biotechnology and medical technology.
Owned/controlled mainly by an external company outside Sweden and a number of foundations in Sweden. Similar to Fortress in the number of companies it currently owns. One difference from Fortress is that it takes higher risk initially and often acquires assets before or after preclinical studies (Fortress explicitly wants to have at least P1 data in humans for the main asset in the pipeline it acquires and builds a subsidiary around), and then with the strategy of selling at proven P2 in order to then reduce the risk of the often financially burdensome P3 and the risk of the later pivotal study (here Fortress wants to be involved all the way if they believes in the data and its potential itself or through partners).
Comments
I mainly monitor the stock market in Scandinavia and North America, and these are the companies/stocks that I found most similar to Fortress Biotech today (please point me to more if there are any). Nevertheless, the structures differ in several points where I feel that there is no direct peer that has purchased medical assets 100% to establish their own subsidiaries around these assets in small management with the most suitable advisory board for each targeted disease or disease group (sometimes only 1-2 drugs in the pipeline per subsidiary). Some of the companies/stocks in Group B have some ownership control either through a high ownership in a specific company or based on the fact that the main owner in their own parent company has strong voting power. But Group B often lacks incentives that Fortress has in a common structure with Group A in revenues partly built up from milestones and royalties. A big advantage of the Fortress model, I believe, is that the total review and control contributes to is that they can allocate their management and financial resources where they are most useful. With mandates, mainly for the private subsidiaries, it has contributed to the fact that they can and have paused/brake (even if they would not admit it themselves) a Ford to simultaneously accelerate a Volvo (I am Swedish after all...). As a co-owner and partner like Group B, it is difficult with such decision-making even with large ownership and a place on the board. You should rather let the money talk in case of possible emissions wherever to place your bet on a "Ford" or "Volvo" if the company is not publicly listed which means that you at least have the opportunity to buy/sell shares in the market, and earns money not in need of new financing. To read potentially negative aspects of Fortress' business model, read under the next point "risks".
9. Risks
First, in relation to this entire text, anyone considering investing money in Fortress Biotech's Common Share and/or Preferred Stock should read the entire "Part 1" (P. 3-68) in the latest Annual Report (2024) where relevant general risks in equity investing are highlighted with vital sector risks and specific risks linked to Fortress Biotech. Although, as I said, I want you to review all of these pages, I will highly recommend many (for fear that you will skip someone), chosen with direct connection to my own review:
P. 4 - Risks Pertaining to Our Existing Revenue Stream from Journey Medical Corporation (“Journey”)
P.5 - Risks Pertaining to our Business Strategy, Structure and Organization, Risks Pertaining to Reliance on Third Parties & Risks Pertaining to Generic Competition and Paragraph IV Litigation
P.7 - Checkpoint and UNLOXCYT
P. 8 - Cyprium and CUTX-101
P. 12 - CAEL-101 (monoclonal antibody for AL amyloidosis)
P. 18 – Competition
P. 19 - Generic Competition & Government Regulation and Product Approval
P. 19-21 - United States Pharmaceutical Product Development Process
P. 22 - United States Review and Approval Process
P. 22-23 - Special FDA Expedited Review and Approval Programs
P. 24 - Orphan Drugs
P. 24-25 - U.S. Marketing Exclusivity and Patent Term Extensions
P. 27 - Pharmaceutical Coverage, Pricing and Reimbursement
P. 28-33 – FDA
P. 34 - Oaktree Agreement (Debt)
P.35-38 - Series A Preferred Stock, R&D, S-3 Shelf & broadly about any future financing needs
P. 38 - Risks Pertaining to Existing Revenue Stream from Journey Medical
P. 40 – Reimbursement
P. 43 - Risks linked to guarantor and/or indemnitor of obligations subsidiaries and partnerships
P. 46-57 - Risks Pertaining to Reliance on Third Parties
P. 58-59 - Risks Pertaining to Legislation and Regulation Affecting the Biopharmaceutical and Other Industries
P. 66 - Fail to comply with the continuing listing standards of Nasdaq
In addition to this, I would like to contribute a personal discussion about potential negative aspects of Fortress Biotech's business model and what it could entail:
Business model - Risks and negative self-perceived perspectives
The disadvantage of the Fortress model may be that they have historically been too dependent on themselves financially and prematurely, despite support from Journey Medical, which contributed to the group's balance sheet early on in its commercialization. Then it is possible to find both advantages and disadvantages of publicly listing some of the subsidiaries that Fortress has done. I have also experienced it historically based on the almost 1.5 years that I have followed Fortress that both Journey Medical and Checkpoint have been traded at a noteworthy discount, probably partly because of the majority control Fortress has/had over decision-making and the annual equity dividend and royalties in Checkpoint's case. In part, I have found this to be understandable based on structure if there was a lack of belief in Fortress as a parent company. However, I must admit that it has sometimes been frustrating to hear other investors' opinions argument on the subsidiaries that they do not want to own based on Fortress incentives, when Fortress in almost all unlisted as listed subsidiaries has largely filled all management and board positions and then to hear investors' thoughts that they do not want to own because of Fortress. Because as I interpret it, Fortress is their subsidiary (a little different in Journey due to the Founders agreement and the strong team from Medicis). They have founded, financed and managed and with that background it should be obvious to be able to see through certain incentives if you have confidence in Fortress management, and can equate and understand the entire connection between them from the start (i.e. started by reading the Founders Agreement).
I understand another side more, that many like me prefer to own the parent company in FBIO/FBIOP which after all still sits on most decisions in the subsidiaries, with a more diversified portfolio/pipeline. I think this has and can contribute to low valuations in Fortress subsidiaries if they are already or are to be listed on the public stock market. And that then contributes to Fortress's value also being kept low given how the public subsidiaries are traded, like a vicious spiral. This at the same time as it can be difficult to clarify the value of the private companies, precisely because they are not daily or hardly at all listed at a price in real time. I can really feel this in subsidiaries like Helocyte, Cyprium and Urica being traded as if they did not exist (based on today's valuation of both Fortress Common Share FBIO and Preferred Stock FBIOP), despite the great value I feel is there. In summary, it can sometimes feel like a Catch 22.
Naively, I have perhaps thought that the Fortress subsidiaries deserve a premium mainly because of the strong management it contributes with and their successes historically. But here one must perhaps understand if investors are partly afraid to own any of their four publicly listed subsidiaries in the belief that Fortress, in control of the A shares, will make decisions that best benefit Fortress' current situation, even though I believe that this is usually in the same interests as the individual shareholder in the specific subsidiary.
Low share turnover/low trading
One last risk I would like to address is the low daily turnover that often occurs in Fortress Common Share (FBIO) and Preferred Stock (FBIOP). However, this mainly applies to Preferred Stock (FBIOP). This can entail risks regardless of whether you want to buy, with artificially increasing prices and risks that the stock becomes overvalued for its underlying value, and on the downside with rapidly falling prices that can mean that you are forced to sell at lower prices than you could have expected before your decision to sell. Another risk, which I personally think is not talked about/written about in general in connection with this, is the volatile share price in stocks like FBIO/FBIOP, when a large/decisive position (in relation to your total economy) has significant daily/weekly/monthly fluctuations. From my own previous experiences, I believe that this can also entail a psychological risk that should not be underestimated.
10. Why (I believe) Fortress Biotech Preferred (FBIOP) will resume its dividend within 1-20 months
I feel that there is a good basis for opportunities for a total value increase of +$500M during these 20 months, which would with margin make Fortress' net income positive. These revenues are built on an incremental model and with it I predict that Fortress will finally reach the snowball-effect that they have long communicated as a target image. I also think that there are already some guarantees for this with the group's two FDA approvals in Q4 2024 for UNLOXCYT, but above all EMROSI whose sales will transform an already commercial portfolio from Journey. With justifiably high expectations, in my opinion, that Checkpoint's buyout will go through in Q2 and CUTX-101 FDA approval in Q3 (PDUFA 30/9), I believe that these guarantees will be confirmed as early as 2025.
There should be good incentives in terms of financial commitments and reputation towards both lending institutions, institutional and private owners in Common Share (FBIO) and Preferred Stock (FBIOP) to reinstate the dividend with a secure balance sheet as a basis to increase the credibility and stability around the company and its two share classes. There should also be incentives among the 9 insiders in the top layer who together own up to 27.9% of Common Share for this to happen as I feel the valuation there has taken a hit as a result of the uncertainty a suspended dividend can bring in a Preferred Stock in an industry in pharma/biotech development, which per se can be perceived as very uncertain.
The incentives should not be that much lower for Founder/President/CEO's Lindsay Rosenwald (other insiders have also bought) when he bought 139,167 shares of the Preferred Stock (FBIOP) worth $2,530,295M in total with an average acquisition value of $18.18 per share (in addition to what he possibly owned from the start). For Rosenwald, where Fortress could reasonably be his "last dance" given his age, I feel that there could be much more than money at stake, because there he should have enough money (the deal for the "excessive" apartment on Manhattan in the press at least testifies to that). Namely, a matter of honour to at least make Fortress stand firm, and not like the remains of Visingsborg (Fortress here in Sweden), in the face of a succession. As the section with "Management" addresses, Rosenwald has been responsible for creating and selling companies in the industry for billions. The same with Michael Weiss (Executive Vice Chairman, Strategic Development at Fortress) whose value of his own shares in his founded TG Therapeutics (TGTX) should amount to quite precisely $600M now (or 6.7 times Fortress Biotech's entire value, but who's counting...). Other insiders and all those who have individually invested primarily in Fortress Private Subsidiaries but also Publicly listed subsidiaries. Rosenwald & Weiss sits on the board and management of almost all of these subsidiaries. For them I believe that the prestige and honour in the phase they both are in life can be at least as big a driving force as money. The same at Journey Medical. We have a management and board of directors full of former high-ranking Medicis employees who ran the company until the day they were acquired, for $2.6B.
In conclusion, I believe that having the S-3 Shelf available, as I mentioned earlier, provides certain guarantees and opportunities, and for that to happen, Fortress must resume dividends on its Preferred Stock.
11. Valuation, Final Conclusions & Position
In every ocean (stock market) or muddy stream (small cap biotech) there is a bottom, and it is possible to argue with a simplified napkin calculation that we could at least see that bottom close to here for Fortress Biotech:
Napkin calculation (2025-05-05)
Cash
CKPT deal close in Q2: $28M (not including CVRs)
Cash Q4: $20.90M (includes non-listed subsidiaries)
Total: $48.90M
Debt
Oaktree debt per Q4 2024: $35.35M (Possibility of $15M more in loans from Oaktree remains)
Fortress does not have to repay more than the interest itself of that $35.35M until March 2027, when the first repayment can be made.
Unpaid $FBIOP dividend: $6.69M
Total: $42.04M
$48.90 (Cash) - $42.04M (Debt) = $6.53M (Net Cash)
Assets
If I only include Fortress ownership in Journey Medical (DERM) and the $6.53M Net Cash:
DERM 44.5%: $72.27M
Net Cash: $6.53M
$72.27M (44.5% of DERM) + $6.53M (Net Cash) = $78.8M (Assets including only their ownership in DERM and net cash)
Market Capitalization
Fortress Biotech Common Share ($FBIO): $54.05M
Fortress Biotech Preferred Stock ($FBIOP): $35.48M
Total: $89.53M
Assets - Market Capitalization
$78.8M (Assets including only their ownership in $DERM and net cash) - $89.53M (Total Market Cap for Fortress FBIO/FBIOP) = -$10.73M
If we had stopped here, it wouldn't have been so promising, right?
But we have two steps left, because if you've made it this far into the text, I know you understand that Fortress Biotech doesn't just consist of a stake in Journey Medical and some net cash. But first, I would like to remind you again of how Journey Medical's future is currently valued today.
ROTH MKH latest Estimated EPS for Journey Medical (April 3, 2025), where it lowered its forecasts for ACCUTANE and QBREXZA given the increased competition mentioned above:
2026: $2.18 EPS
2027: $3.32 EPS
2028: $5.18 EPS
P/E based on todays $7.03 (2025-05-05) share price:
2026: P/E 3.22
2027: P/E 2.11
2028: P/E 1.35
And by 2028, EMROSI should reasonably have a few years left until its peak in global sales.
Now for the assets not included above. A brief summary:
Asset 1 – Urica Therapeutics
Ownership: 69.6%
Annual Equity Dividend: 2.5%
Uricas ownership in Crystalys: 35%
Royalty: 3% on future net sales of Crystalys Dotinurad
Asset 2 - Cyprium
Ownership: 73.1%
Annual Equity Dividend: 2.5%
Milestones: Up to $129 million in aggregate development and sales milestones
Royalties (on every annual sale):
3.00% of net sales up to $75M
8.75% between $75M and $100M
12.50% over $100M
PRV sale: Potential~$150M
Asset 3 - Checkpoint (deal already included, only CVR’s & Royalties here)
CVR:
$0.20 - $1.384M
$0.45 - $3.115M
$0.70 - $4.846M
Royalties: 2.5%
Asset 4 - Caelum (Sold)
CAEL-101 FDA Approval: $19.6M
CAEL-101 Milestones: Eligible to $124.6M in future milestones
Asset 5 - AEVITAS (Sold)
Milestones: Eligible to ~$140M in potential late-stage development, regulatory and sales milestone payments plus royalties from 4D-175
Asset 6 – Helocyte
Ownership: 83%
Annual Equity Dividend: 2.5%
Royalty: 4.5%
Asset 7 - Cellvation
Ownership: 79.2%
Annual Equity Dividend: 2.5%
Royalty: 4.5%
Asset 8 - Oncogenuity
Ownership: 73,5%
Annual Equity Dividend: 2.5%
Royalty: 4.5%
Asset 9 - Avenue
Ownership: 9.2%
Annual Equity Dividend: 2.5%
Royalty: 4.5%
Asset 10 – Mustang
Ownership: 6.3%
Annual Equity Dividend: 2.5%
Royalty: 4.5%
Again, with these 10 assets not included in the calculation:
-$10.73M
That -$10.73M is seen in a different light (or pure darkness, depending on perspective...) now, isn't it?
With what I believe is undervalued Journey Medical in the calculation, the market today values the remaining assets in Fortress Biotech at $10.73M. An average of $1.073M per asset of these 10.
1. I think Journey Medical (DERM) is undervalued today
2. I think these "10 Assets" are worth more than $10.73M
3. The sum of answers 1 and 2 makes me think Fortress Biotech overall, including both Common Share (FBIO) and Preferred Stock (FBIOP), is undervalued today
And yes, yes, I know. Just because you can demonstrably sign the world's best football (yes, FOOTBALL) player of all time on a napkin, doesn't mean you can or should do a complete financial analysis on a napkin. But as I've emphasized, this is not a financial analysis. I lack both the knowledge and the experience for that. This document/post is a review of how I thought, reasoned and saw opportunities as risks with my personal investment. If I can't describe to myself briefly (well...), on a napkin (well...) why I believe in a single investment after much research, then I must at least actively try to pass.
Conclusion
In Fortress I find much of what I normally look for in an investment. A small, undiscovered company with significantly undervalued diversified assets, backed by management with a successful history and strong incentives in the form of high insider ownership.
I see significant chances for a reinstated in and maintained dividend in the foreseeable future for Fortress Biotech Preferred (FBIOP). I also see a stratospheric opportunity for Fortress Common Share (FBIO) shareholders to experience significantly higher share prices in the future.
If (when) Fortress announces that they will reinstate the dividend on their Preferred Stock (FBIOP), I believe that the stock will rise above $25.00 (Liquidation Preference Price) by the next X-day given that as of today when I write this in 2025-05-05 are 10 accumulated dividends (10 x $0.1953 = $1.953, 29.50% yield today) that can then be paid to shareholders of the stock. In my segment "Dividend" here I also show that for the entire time period for my review of a maximum of 20 months in December 2026 there are a total of 30 dividends worth $5.8590 in dividend per share, which at today's share price of $6.61 corresponds to a dividend yield of 88.64%. Personally, I hope and believe that I can own the stock significantly longer than that given that I believe that Fortress' "snowball" is in full swing before the start of 2027. I feel that with high dividends, given my large position in my total portfolio, I can create my own Fortress "snowball" to reinvest the dividends in either more Preferred or Common Shares as a unique opportunity for me.
The unique opportunity to get back almost all your invested money in dividends over a period of 20 months is no less when you add the increase in value of your Preferred from today's level of $6.61. At $25.00 (Liquidation Preference Price) is an upside of 278%. As I wrote, I believe the stock will initially go up more than that, but after the first X-day of accumulated dividends I think it will fall below $25 and then over time stabilize in the range of maybe between $22-$24 per share, which would correspond to an upside of 233%-263%, in addition to your accumulated dividends.
If so, when do I think the dividend will resume?
Personally, I think it is harder to predict that question. But in addition to following EMROSI's sales launch, development and possible partner deals outside the US, I will have my sights set on a couple of key triggers and their timing:
1. Sun Pharma deal to buy Checkpoint closes (expected now in Q2). Clear signs after the vote at the Shareholders' Meeting on May 28 mean that the interest and opportunity ahead of Fortress Biotech's Annual Meeting on June 17 increases significantly. Also, keep an eye on any CVR assigned from the deal.
2. CUTX-101 PDUFA on September 30. An approval would secure many years of dividend value in the long term for Fortress Biotech.
3. CAEL-101 P3a-b data readout during H2 in 2025. In the event of a favourite in a repeat from the 2024 study results update, ASH's Annual Meeting/Conference on December 6-9 should be an extremely relevant dates and opportunity.
4. If point 3 is positive, a new PDUFA date should be in place in Q2-Q3 next year 2026.
Given the sum of parts in this review/analysis, with all the cannonballs Fortress has in its arsenal to hit the target with, I think a more reasonable valuation would be in the range of $12.50-$15.00 today for Fortress Biotech Preferred Stock FBIOP even though a decision on resuming dividends has not been made. The odds for a successful 2025-2026 and the dividends already accumulated should, in my opinion, appeal to that extent. Even a possibly unsuccessful context during these two years should not mean that the dividends are lost with a delay into 2027.
Current position/holdings of the mentioned stocks in the post:
Fortress Biotech Preferred Stock (FBIOP) – Shareholder since July 2024. Large holding.
Fortress Biotech Common Share (FBIO) – Shareholder since February 2024. Large holding.
Journey Medical (DERM) – Shareholder since December 2024. Medium holding.
Checkpoint Therapeutics (CKPT) – Shareholder since April 2024. Small holding.
Avenue Therapeutics (ATXI) – No Position.
Mustang Bio (MBIO) - No Position.
TG Therapeutics (TGTX) – Shareholder since January 2025. Minimal position mostly to follow M.W. (too poorly knowledge in the company today for a larger position)
Royalty Pharma (RPRX) - No Position.
Ligand Pharmaceuticals (LGND) - No Position.
DRI Healthcare Trust Units – (DHT.UN) - No Position.
XOMA Royalty (XOMA) - No Position.
Linc (LINC.ST) - No Position.
Flerie (FLERIE.ST) - No Position. On the watchlist since worked on this analysis.
Karolinska Development (KDEV.ST) - No Position. On the watchlist since worked on this analysis.
Do me a favour and read the “Disclaimer” in the very beginning again!
Then I want to thank my two idols Cluseau because he is Top 1, and because I stole the structure from the introduction and "Table of Contents" and MDC/Clark Street Value because his blog posts over the years in the sector have taught me the most.
MDC/Clark Street Value - https://clarkstreetvalue.blogspot.com/
I welcome comments and corrections about errors at zellchair@gmail.com.
/Zellchair